Method Article
Sexual Crosses with the Mucoromycete Phycomyces blakesleeanus
In This Article
Summary
Here we present a basic protocol for the induction of P. blakesleeanus mating.
Abstract
Phycomyces blakesleeanus, a filamentous fungus within the Mucoromycota phylum, is distinguished by its remarkable capacity for environmental perception and adaptive responses. While past work has shown that environmental stimuli, including gravity, light, moisture, and nutrient availability, influence its growth dynamics and reproductive strategies, the underlying mechanisms remain a focal area of research. Environmental cues trigger sexual or asexual reproduction. Sexual reproduction begins with pheromone signaling, which triggers hyphal chemoattraction, eventually leading to serial morphological transitions culminating in the formation of a zygospore.
In a laboratory setting, crosses of P. blakesleeanus result in complementary mycelia undergoing the sexual cycle at different stages. Our work aims to test if environmental cues can trigger mating across the mycelia of P. blakesleeanus. Crosses of P. blakesleeanus grown on nutrient-limited media will be subjected to nutrient-limited agar to trigger a sexual response in accordance with nutrient deprivation. Successful triggering of mating in P. blakesleeanus will facilitate future studies that require a large amount of sexually reproducing mycelia in specific stages. The outcome of this research will further enhance our understanding of how P. blakesleeanus reproductive mechanisms are influenced by environmental factors, contributing to the broader knowledge base on the sexual reproduction of filamentous fungi.
Introduction
The induction of mating in fungal systems in laboratory conditions has advanced the understanding of eukaryotic genetics, cell biology, evolutionary biology, and biotechnology. The dikaryan fungi, in particular the Ascomycota, feature the most extensive knowledge about mating induction in a laboratory setting1. Interestingly, the first fungus in which sexual reproduction was proposed, Syzygites megalocarpus, is not a member of Ascomycota, but rather a member of the phylum Mucoromycota2. The phylum Mucoromycota is an early-diverging group formerly classified as "Zygomycota" but now considered sister to the Dikaryan lineages3,4. The Mucoromycota, along with Zoopagomycota, are evolutionarily significant as these phyla represent the transition of fungi to terrestrial ecosystems4,5. Like other fungi, the Mucoromycetes use asexual and sexual reproductive strategies in response to their environment3,6. Under nutrient-limited conditions, mucoromycetes will begin the sexual cycle6. Mucoromycetes exhibit both homothallic and heterothallic mating mechanisms, where compatibility is determined by the mating-type genes sexM and sexP, designated as (-) and (+) respectively7,8,9.
Blakeslee10 emphasized the sensitivity of the mucoromycete sexual cycle to external conditions, noting that moisture is critical for their formation and that nutrient availability in the substrate plays a significant role. Complementary mating types begin the sexual cycle through cooperative synthesis of trisporic acids (TA) using beta-carotene as a precursor11,12,13. Following the detection of TA, the responding vegetative hyphae thicken and become highly branched zygophores14,15,16. The zygophores continue production of TA and mutually chemoattract. In P. blakesleeanus, zygophores differentiate within the substrate and are not readily visible on solid agar media17. Upon making contact, the zygophores intertwine and become aerial zygophores.
As the sexual cycle continues, the tips of the zygophores attach, and the middle of the cells push out to form a ring-like structure with coralloid swelling at the base, completing the transition to the progametangium. The tips of the cells forming the progametangium begin to fuse and develop into the gametangium. At the gametangia stage, the zygophores display thorn-like ornamentation. The cell wall at the tips dissolves and adventitious septa appear, delimiting the area where the zygospore will form, and the zygophores act as tong-like suspensors18. The zygospore will become pigmented as its cell wall thickens and it acquires additional thorn-like ornamentation17,18. Once formed, the zygospore will enter a period of dormancy before restarting the growth cycle.
Phycomyces blakesleeanus is a heterothallic mucoromycete notable for its large cells and environmentally responsive sporangiophores6,8,18. This organism is easily cultivated in the lab and the portions of the sexual cycle leading to the formation of zygospores can be observed in the span of 8-10 days. As a model, P. blakesleeanus has been examined for its capability to sense light in its environment17,19. The ease of cultivation and the capacity to induce mating also made it an ideal model to study the mechanism behind its ability to perceive light20; these findings also highlighted the evolutionary conservation of light-sensing mechanisms in fungi. In P. blakesleeanus, light has been shown to inhibit sexual reproduction via these conserved light-sensing proteins21. Recent evolutionary developmental studies have sought to understand what genes are contributing to cell differentiation during the P. blakesleeanus sexual cycle6. Correlating morphogenesis to specific genes would require that sufficient tissue of the same cell types be isolated to perform gene expression studies.
While protocols for inducing mating of P. blakesleeanus in a laboratory setting have been previously described, some only mention the type of medium to use and the relevant strains18. Some protocol descriptions do not include specific media formulation but do describe where to position presumptive complementary mating types on a plate10. More recent protocols allow for increased production of zygospores by either mixing spores of each mating-type and inoculating with the mixed spore suspension21 or by placing mating types some distance apart and letting the culture incubate for 20 days22. These approaches are useful for generating ample differentiated cells, specifically zygospores, but may not be suitable for observing a developmental time course or selecting sexual structures that form prior to zygospores for single-cell transcriptomics. Other work has addressed this by placing complementary mating types distance apart on solid media to allow the observation of serial morphological transitions as the sexual cycle begins6,22,23. As is the case with other fungi, P. blakesleeanus mycelia expands radially24,25. Therefore, when complementary mating types are grown on the same plate, different portions of their mycelia will come in contact at different times. Since contact between mycelia is among one of the first steps in P. blakesleeanus sexual cycle, this means that different portions of interacting mycelia will be at different stages in the sexual cycle. This asynchrony could impact the outcome of a gene expression study, such that if differentiated cell types are mixed and if they do have distinct gene expression programs, then it would be difficult to ascribe the role of a gene to one particular structure.
In addition to the value of P. blakesleeanus as a model for examining genes involved in morphogenesis during the sexual cycle, its vigorous growth and capacity to differentiate in the span of a few days make it an ideal fungus for training students interested in early-diverging filamentous fungi and for use in an undergraduate classroom setting to learn about the diversity of fungi and their developmental processes. The protocol presented here makes use of three concentrations of two different types of media to demonstrate the effect of nutrient availability on mycelium appearance, the induction of mating, and enrichment for particular sexual structures, either for quantifying, observation, or potential single-cell transcriptomics.
Protocol
1. Media preparation
- Suspend powdered cornmeal agar (CMA) or potato dextrose agar (PDA) in deionized water. For 100% CMA or PDA, follow the manufacturer's instructions and suspend 17 g of CMA in 1 L of deionized water or 39 g of PDA in 1 L of deionized water. For N% CMA or PDA, suspend 1/N of the manufacturer-recommended amount and supplement with additional agar to achieve an agar concentration of 7.5% (w/v).
NOTE: Optional step: Prior to autoclaving, add 25 µg/mL of chloramphenicol if bacterial contamination is a concern. - Sterilize media in an autoclave, at 121 °C for 15 min.
- Cool the media to ~60 °C by placing it for 30 min in a water bath set to 60 °C.
NOTE: If the warm bottle of media can be held for 6 s with little to no discomfort, then it is ready to pour. - Pour plates under aseptic conditions in a laminar flow hood or biological safety cabinet. Tilt the bottle of media over an open, empty Petri dish and pour only enough to completely cover the bottom.
- Alternatively, use a glass serological pipette and pipette 20 mL of media into each plate.
NOTE: This approach increases the time that the media bottle is at a lower temperature and raises the risk of prematurely solidifying the agar.
- Alternatively, use a glass serological pipette and pipette 20 mL of media into each plate.
- Allow the media to resolidify. Store the plates at 4 °C if not used immediately.
2. Preparing fungal spores or tissue
- Obtain cultures of each P. blakesleeanus mating type (e.g., NRRL 1555 (-), NRRL 1554 (+), NRRL 1464 (-), and NRRL 1465 (+)). To inoculate crosses with spores, first grow each mating type in pure culture on 100% CMA or PDA
- Cut a portion of leading-edge mycelia from existing cultures and place the excised mycelium on a 100% CMA/PDA plate. Incubate pure cultures for 1 week at 27 °C under a 12 h light cycle.
- Once sporangiophores are present, flood a sporulating pure culture with 0.01% Tween 20 in sterilized, deionized water using aseptic technique. With a P1000 micropipette, draw 1.0 mL of the Tween 20-spore mixture from the plate and into a microcentrifuge tube.
- Centrifuge in a mini centrifuge for 30 s, then decant the supernatant.
- If more spores are needed, continue to add 1.0 mL of the Tween 20 spore mixture into the same microcentrifuge tube and repeat step 2.4. After obtaining a suitable amount of spores, decant the supernatant and replace it with 500 µL of sterile deionized water.
- Optional: Use a hemacytometer to estimate the concentration of spores, which will inform whether more water needs to be added or if the spores need to be re-centrifuged.
- Alternatively, inoculate the crosses with leading-edge mycelia from the pure cultures.
- Use a sterilized razor blade or cork hole borer to excise the tissue and plate the inoculum immediately.
- Set up the crosses as 2-way crosses, 4-way crosses, or 8-way crosses (Figure 1).
NOTE: If crosses are being set up for transcriptomic work, it is recommended to set up 1-2 additional plates per condition/media to serve as indicator plates. Indicator plates allow investigators to limit light exposure to experimental plates, as light inhibits sexual reproduction in P. blakesleeanus.- For a 2-way cross, place complementary mating types opposite each other (Figure 1A).
- Place the mycelia of the (-) mating-type, NRRL 1555 or NRRL 1464, at least 1 cm away from the edge of a Petri dish on PDA or CMA.
- On the same plate, opposite of where the (-) mating-type was placed and 1 cm away from the edge of the Petri dish, place the (+) mating-type, NRRL 1554 or NRRL1465 (Figure 1A).
NOTE: The distance between complementary mating types will influence the size of the tissue participating in the sexual cycle. Mating types further apart allow the mycelia to expand more, which will increase the number of sexual interactions between the two partners.
- For a 4-way cross, place like mating types opposite each other but neighboring a complementary mating type (Figure 1B).
- Place the spores or mycelia of the (-) mating types, NRRL 1555 and NRRL 1464, opposite each other at least 1 cm away from the edge of a Petri dish on PDA or CMA.
- On the same plate, choose a location between the two (-) mating types and at least 1 cm away from the edge of the plate and inoculate the site with spores or mycelia of the (+) mating type, NRRL 1554 or 1465.
- On the same plate, opposite where the first (+) mating type was inoculated and at least 1 cm away from the plate, place the other (+) mating type.
- For an 8-way cross, alternate mating types along the periphery of the Petri dish, featuring four of each mating type (Figure 1C).
- For a 2-way cross, place complementary mating types opposite each other (Figure 1A).
- After inoculating, seal the plates with parafilm or (leave them unsealed) and place them in a secondary container prior to incubating at 22 °C in the dark. Observe the plates daily for evidence of mating during which time, take photographs from underneath the plate and trace the mycelia as the cultures grow.
NOTE: After 24 h post inoculation, expect to see mycelia expanding on all media types but individuals are not likely to be in contact at this stage. - Depending on the media type (PDA or CMA) and the formulation (25%, 50%, 100%), if the mycelia begin to make contact, inspect mycelia of complementary mating types that are in contact under a dissecting scope for evidence of mating. Take photos of interacting mycelia with a camera or a smartphone mounted to the dissecting scope.
- Acquire images of mycelia and stitch them together (e.g., using Panorama Stitcher application). Use ImageJ to estimate the area of the mycelia (see online tutorials).
Results
Following a 4-way cross, each strain varied slightly in its growth rate as determined by the change in area of the mycelium (Figure 2). While not statistically significant, NRRL 1555 had a faster change in area when plated on 25% CMA, 25% PDA, or 100% PDA. Similarly, NRRL 1465 had a higher change in area when grown on 50% PDA or CMA. NRRL 1464 had the quickest increase in area when grown on 100% CMA. After 24 h (1 DPI), strains growing on PDA appeared more yellow relative to the strains grown on CMA (Figure 3)-PDA has previously been shown to increase pigment production26. Strains on 100% PDA had, on average, a higher measure of area after 24 h (Table 1). This is in contrast to 25% CMA, which had the smallest average area at 1 DPI (Table 1).
Contact between neighboring strains was not observed until 2 DPI and only among strains grown on PDA, especially 100% PDA (Figure 4). Mating was observed on 100% PDA at 2 DPI. The strains grown on CMA did not make contact at 2 DPI, though 100% CMA featured strains that grew very near each other (Figure 4). After 3 days of incubation (3 DPI), strains grown on all formulations of PDA exhibited mating, although the density of differentiated cell types differed (Table 2 and Figure 5). One replicate of 25% CMA and one replicate of 50% CMA also exhibited mating (Table 2); the remaining CMA plates did not exhibit any mating (Table 2). Interestingly, the strains grown on PDA appeared to have denser, yellow mycelia and aerial hyphae, which could have been developing into sporangiophores (Figure 5). By 4 DPI, the PDA plates were exhibiting mating reactions alongside asexual sporangiophores (Figure 6). The CMA plates had strains with more diffuse mycelia and fewer sporangiophores (Figure 6). Mating was not observed on 100% CMA (Table 2).
Representative plates were observed under a dissecting scope at 4 DPI to assess whether cell types associated with the beginning of the sexual cycle could be easily observed. In general, a decreasing concentration of nutrients was correlated with qualitatively less dense, differentiated, sexual mycelia (Figure 7 and Figure 8). When grown on 100% PDA, individual aerial zygophores or progametangia could not be visualized and appeared as a mass of cells (Figure 7A). While 50% and 25% PDA appeared to have lower cell density at the site of mating, the 25% PDA plates featured cells that could be easily distinguished (Figure 7B,C). When comparing the types of cells observed in 50% PDA to 25% PDA, those at the lower concentration appeared to be predominantly the same type, whereas at the higher concentration, there was a mix of cells at different stages (Figure 7D,E). Notably, with 50% PDA, the cells observed at 4 DPI included aerial zygophores that had just emerged (Figure 7D, asterisk) and some that were transitioning to progametangia (Figure 7D, arrow). With 25% PDA, most cells seemed to be at very similar developmental stages (Figure 7E).
Plates of the various concentrations of CMA were assessed alongside the PDA plates at 4 DPI (Figure 8). As previously mentioned, mating was not observed when 100% CMA was used (Figure 8A). At either 50% CMA or 25% CMA, mating was observed and the overall number of cell types associated with sexual reproduction was lower (Figure 8B,C). The aerial zygophores observed at 4 DPI appeared to be the same size and height across the zone of interaction (Figure 8B,C). Given the lower overall number of cells participating in the sexual cycle, these could hypothetically be excised with sterilized razor blades or dissecting needles and immediately frozen in liquid nitrogen for downstream RNA isolation. Being able to select specific structures at the same developmental time point may increase the robustness of gene expression studies seeking to connect morphological changes to genes.
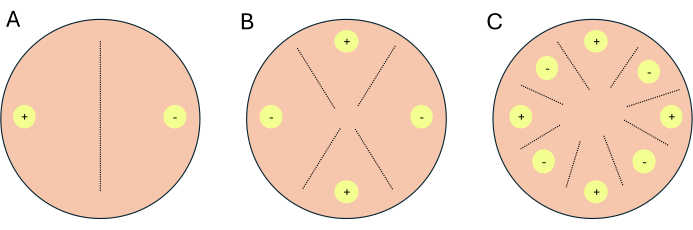
Figure 1: Schematic of three cross formats. (A) A 2-way cross with two inoculation sites (yellow circles) with complementary mating types (+) and (-). (B) a 4-way cross with two inoculation sites of (+) mating-type (yellow circles with +) and two inoculation sites of (-) mating -type (yellow circles with -). (C) An 8-way cross with alternating inoculation sites (yellow circles) of complementary mating-types (+) and (-). The dashed lines represent the predicted site of mating interactions. Please click here to view a larger version of this figure.
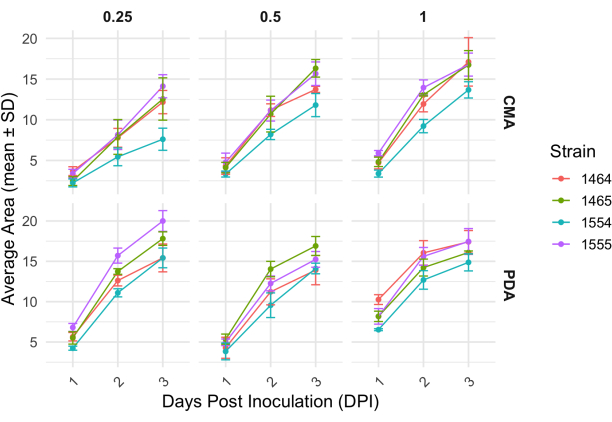
Figure 2: Change in area over time for each strain on CMA and PDA at different concentrations. The average area of P. blakesleeanus colonies was plotted over 3 days post inoculation. Error bars represent the standard deviation of the mean area. Also see Table 1. Plots were generated with ggplot227. Abbreviation: DPI = days post inoculation. Please click here to view a larger version of this figure.
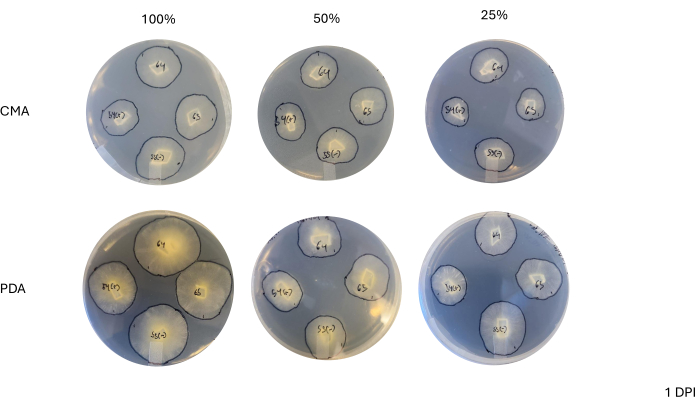
Figure 3: Representative images of 4-way crosses at 1 day post inoculation. Comparison of colonies shows differences across media type and formulation, highlighting that colonies on 100% PDA appear more yellow than CMA and that growth appears faster on 100% PDA. Images were taken from the bottom of the Petri dish. Black lines were drawn with a marker to outline the mycelia. Abbreviations: CMA = cornmeal agar; PDA = potato dextrose agar; DPI = days post inoculation. Please click here to view a larger version of this figure.
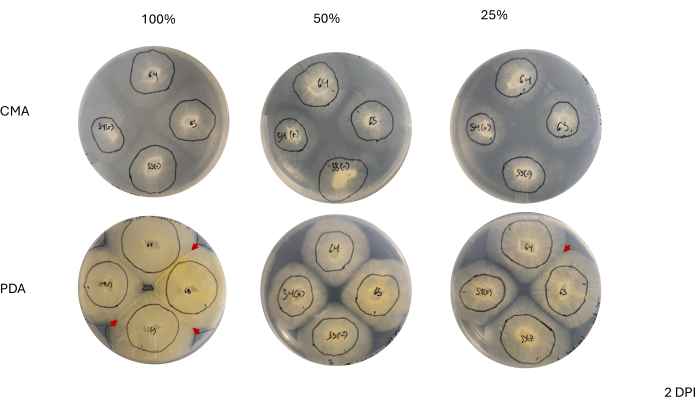
Figure 4: Representative images of 4-way crosses at 2 days post inoculation. Comparison of colonies highlights the apparent density of mycelial growth, where colonies on PDA appear to have a denser mycelium than those on CMA. Additionally, colonies on PDA appear more yellow and most have either made contact or are nearing the point of contact. The red arrowheads highlight areas where mating interactions have started, as indicated by an increase in tissue density. This is in contrast to colonies on CMA where only those on 100% CMA are nearing contact, and all CMA formulations feature colonies that are relatively less yellow compared to those on PDA and none have started mating. Images were taken from the bottom of the Petri dish. Black lines were drawn with a marker to outline the mycelia. Abbreviations: CMA = cornmeal agar; PDA = potato dextrose agar; DPI = days post inoculation. Please click here to view a larger version of this figure.
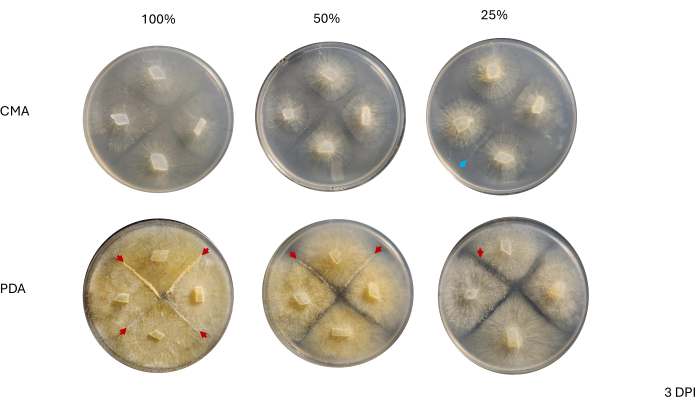
Figure 5: Representative images of 4-way crosses at 3 days post inoculation. Comparison of colonies between the two media types highlights that mycelial density is higher when grown on PDA compared to CMA. The red arrowheads highlight that all PDA plates host at least one site of mating interactions. The colonies on 100% CMA and 50% CMA have not made contact, but two colonies on 25% CMA (blue arrow) have made contact. Images were taken from above the Petri dish. Abbreviations: CMA = cornmeal agar; PDA = potato dextrose agar; DPI = days post inoculation. Please click here to view a larger version of this figure.
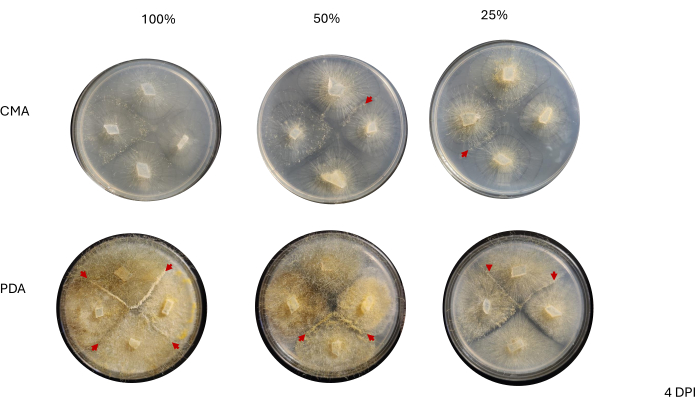
Figure 6: Representative images of 4-way crosses at 4 days post inoculation. All colonies on PDA host at least one mating interaction (red arrows) and display a yellow mycelium. Additionally the colonies on PDA show extensive growth of aerial hyphae (i.e. asexual sporangia) that may impede observation of mating interactions. The colonies on 50% and 25% CMA also exhibit evidence of mating interactions (red arrowheads) and have less aerial hyphal growth compared to those on PDA. Images were taken from above the Petri dish. Abbreviations: CMA = cornmeal agar; PDA = potato dextrose agar; DPI = days post inoculation. Please click here to view a larger version of this figure.
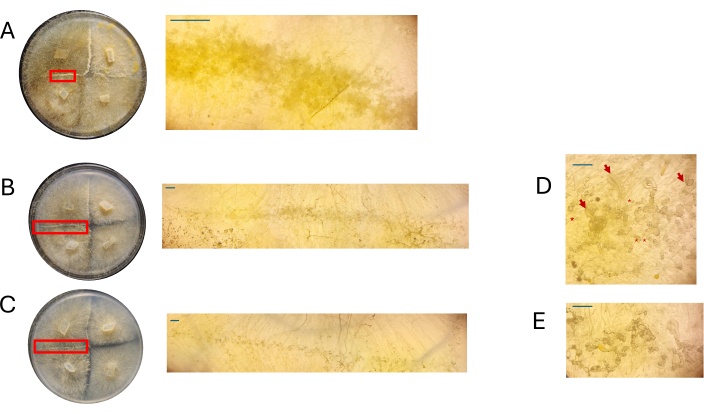
Figure 7: Images of crosses on PDA. (A) 100% PDA, (B) 50% PDA, and (C) 25% PDA. Regions of interest were magnified (red rectangles). All images were acquired at 4 DPI. An additional view of (D) 50% PDA highlights the presence of sexually reproducing cells at different stages: young aerial zygophores: asterisks; older aerial zygophores: red arrows. (E) 25% PDA exhibits sexually reproducing cells at different stages, though this representative view shows a less crowding of these structures compared to 50% PDA. Scale bars = 1 mm (A-C), 0.5 mm (D,E). Abbreviations: PDA = potato dextrose agar; DPI = days post inoculation. Please click here to view a larger version of this figure.
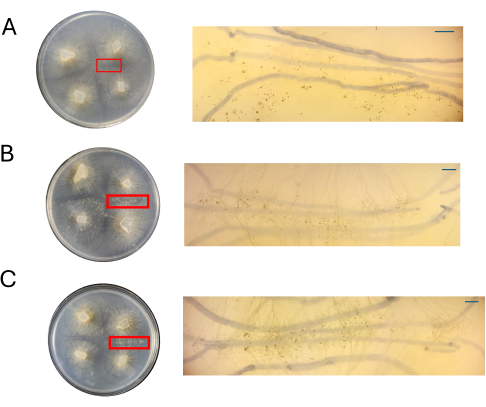
Figure 8: Images of crosses on CMA. (A) 100% CMA, (B) 50% CMA, and (C) 25% CMA with magnification of regions of interest. Distinct sexual structures are readily observed for 50% (B) and 25% (C) CMA, and crowding is minimal. No sexual structures are seen on 100% CMA at this timepoint. All images were acquired at 4 DPI. Petri dish images and dissecting scope images were obtained from above the Petri dish. The dark lines in the backgrounds of the dissecting scope images are drawn with a black marker that was used to track the expansion of the mycelium. Scale bars = 1 mm. Abbreviations: CMA = cornmeal agar; DPI = days post inoculation. Please click here to view a larger version of this figure.
Table 1: Area of mycelia for each strain over 4 days. Please click here to download this Table.
Table 2: Pairwise assessment of contact and mating for each strain on different media. 0 = No, 1 = Yes. Please click here to download this Table.
Discussion
A simple protocol for inducing sexual reproduction in P. blakesleeanus in a laboratory setting is presented here. One of the most critical considerations for this protocol is nutrient limitation. It is hypothesized that fungi co-opted sexual reproduction as a response to harsh environmental conditions such as nutrient limitation6,28,29,30,31,32. Varying the level of nutrients can inform experimental design. For example, if a researcher is interested in comparing the genes expressed in aerial zygophores versus gametangia, then a more extreme nutrient limitation (such as 25% CMA) would be recommended as the density of sexual structures will be less compared to higher formulations of CMA or PDA. A lower density of sexual structures, such as what was observed with 50% or 25% CMA means that isolation of a specific differentiated cell type would not only be easier (as the cells would be easily identified and excised) but would also ensure that only the cell type of interest is sampled.
Trainees or students would also benefit from this protocol as it enables this group to track cell differentiation and reliably distinguish between the various cell types that develop during the P. blakesleeanus sexual cycle. The use of CMA and PDA reflects prior work on the induction of mating of mucoromycetes18,22,23, and powdered forms are available for purchase, making them among the more accessible choice of media in a training or educational setting compared with other synthetic media.
Since the goal of this protocol is ultimately to demonstrate development and differentiation, and it has been pointed out that nutrient concentration impacts whether distinct cell types can be easily observed, a critical step in this protocol is ensuring the appropriate media composition (protocol section 1). If additional agar powder is not added to diluted PDA or CMA, the media may not solidify properly, and subsequent mating interactions may not be easily observed. Additionally, close attention should be given to the placement of complementary and like mating types (protocol section 2). As P. blakesleeanus is heterothallic, it requires a complementary mating partner, and if that partner is inaccessible or a like mating-type is used instead, then mating will not occur10,33,34.
A limitation of this protocol is the asynchronous development of sexual structures. P. blakesleeanus, like other fungi grown on solid media, expands radially in all directions. This means that portions of the mycelium will be at more advanced stages of the sexual cycle than others. Protocols exist to synchronize sexual development in Ascomycetes35, but these have not been successfully adapted to Mucoromycetes. This means that work investigating gene expression during the sexual cycle will be subject to noise if too many cells are at different developmental stages. Given the observations in this study, it would appear that while limiting nutrient availability leads to fewer overall sexually reproducing cells, the cells that are observed appear to be at similar stages of development. Therefore, limitation of nutrients may help limit the density of differentiated sexual structures, granting a researcher access to mostly-synchronous tissue at the expense of lower yields.
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgements
We would like to thank the Colorado College Natural Sciences Executive Committee, the Organismal Biology and Ecology Department, and the Hevey Family Fund for Student Research for funding this work. We also extend our gratitude to Alice Keller and Tia Hutchens for their technical support.
Materials
| Name | Company | Catalog Number | Comments |
| Agar powder | ThermoFisher | 3453PK | |
| chloramphenicol | FisherSci | BP904-100 | |
| Cornmeal agar | Carolina | 742460 | |
| Panorama Stitcher | Apple App Store | ||
| Phycomyces blakesleeanus (-) NRRL 1555 | United States Department of Agriculture - Agricultural Research Service | 1555 | USDA-ARS will only ship to academic researchers; see below for alternatives |
| Phycomyces blakesleeanus (-) NRRL 1564 | United States Department of Agriculture - Agricultural Research Service | 1564 | USDA-ARS will only ship to academic researchers; see below for alternatives |
| Phycomyces blakesleeanus (-) Tube Culture | Carolina Biological Supply | 156183 | Education-grade culture available for purchase; NRRL 1555 |
| Phycomyces blakesleeanus (+) NRRL 1554 | United States Department of Agriculture - Agricultural Research Service | 1554 | USDA-ARS will only ship to academic researchers; see below for alternatives |
| Phycomyces blakesleeanus (+) NRRL 1565 | United States Department of Agriculture - Agricultural Research Service | 1565 | USDA-ARS will only ship to academic researchers; see below for alternatives |
| Phycomyces blakesleeanus (+) Tube Culture | Carolina | 156182 | Education-grade culture available for purchase; NRRL 1554 |
| Potato dextrose agar | FisherSci | DF0013-17-6 | |
| Sprout Plus Mini Centrifuge | Heathrow Scientific | SKU 120610 | |
| Tween 20 | Sigma-Aldrich | P6585-10ML |
References
- NaranjoOrtiz, M. A., Gabaldón, T. Fungal evolution: diversity, taxonomy and phylogeny of the fungi. Biol Rev Camb Philos Soc. 94 (6), 2101-2137 (2019).
- Idnurm, A. Sex determination in the firstdescribed sexual fungus. Eukaryot Cell. 10 (11), 1485-1491 (2011).
- Spatafora, J. W., et al. A phylumlevel phylogenetic classification of zygomycete fungi based on genomescale data. Mycologia. 108 (5), 1028-1046 (2016).
- Wang, Y., et al. Divergent evolution of early terrestrial fungi reveals the evolution of mucormycosis pathogenicity factors. Genome Biol Evol. 15 (4), evad046(2023).
- Gryganskyi, A. P., et al. Sequencing the genomes of the first terrestrial fungal lineages: what have we learned. Microorganisms. 11 (7), 1830(2023).
- Peña, J. F. The evolutionary developmental biology of Mucoromycotina [Doctoral dissertation]. , University of California, Riverside. (2022).
- Wetzel, J., Burmester, A., Kolbe, M., Wöstemeyer, J. The matingrelated loci sexM and sexP of the zygomycetous fungus Mucor mucedo and their transcriptional regulation by trisporoid pheromones. Microbiology (Reading). 158 (Pt 4), 1016-1021 (2012).
- Camino, L. P., Idnurm, A., CerdáOlmedo, E. Diversity, ecology, and evolution in Phycomyces. Fungal Biol. 119 (11), 1007-1021 (2015).
- Gryganskyi, A. P., et al. Structure, function, and phylogeny of the mating locus in the Rhizopus oryzae complex. PLoS One. 5 (12), e15273(2010).
- Blakeslee, A. F. Sexual reproduction in the Mucorineae. Proc Am Acad Arts Sci. 40, 205-319 (1904).
- Sutter, R. P., Grandin, A. B., Dye, B. D., Moore, W. R. (-) mating typespecific mutants of Phycomyces defective in sex pheromone biosynthesis. Fungal Genet Biol. 20 (4), 268-279 (1996).
- Sahadevan, Y., RichterFecken, M., Kaerger, K., Voigt, K., Boland, W. Early and late trisporoids differentially regulate βcarotene production and gene transcript levels in the mucoralean fungi Blakeslea trispora and Mucor mucedo. Appl Environ Microbiol. 79 (23), 7466-7475 (2013).
- Gooday, G. W., Carlile, M. J. The discovery of fungal sex hormones: III. Trisporic acid and its precursors. Mycologist. 11 (3), 126-130 (1997).
- Banbury, G. H. Processes controlling zygophore formation and zygotropism in Mucor mucedo Brefeld. Nature. 173, 499-500 (1954).
- Sutter, R. P., Whitaker, J. P. Zygophorestimulating precursors (pheromones) of trisporic acids active in (-)Phycomyces blakesleeanus. Acidcatalyzed anhydro derivatives of methyl 4dihydrotrisporateC and 4dihydrotrisporateC. J Biol Chem. 256 (5), 2334-2341 (1981).
- Gooday, G. W. Functions of trisporic acid. Philos Trans R Soc Lond B Biol Sci. 284, 509-520 (1978).
- Bergman, K., et al. Phycomyces. Bacteriol Rev. 33 (1), 99-157 (1969).
- O'Donnell, K. L., Hooper, G. R., Fields, W. G. Zygosporogenesis in Phycomyces blakesleeanus. Can J Bot. 54, 2573-2586 (1976).
- Delbrück, M., Reichardt, W. I. System analysis for the light growth reactions of Phycomyces. Cellular Mechanics in Differentiation and Growth. Rudnick, D. , Princeton University Press. 3-44 (1957).
- Idnurm, A., et al. The Phycomyces madA gene encodes a bluelight photoreceptor for phototropism and other light responses. Proc Natl Acad Sci USA. 103 (12), 4546-4551 (2006).
- Shakya, V. P. S., Idnurm, A. The inhibition of mating in Phycomyces blakesleeanus by light is dependent on the MadAMadB complex that acts in a sexspecific manner. Fungal Genet Biol. 101, 20-30 (2017).
- Yamazaki, Y., Miyazaki, A., Kataoka, H., Ootaki, T. Effects of chemical components and nitrogen sources on zygospore development in Phycomyces blakesleeanus. Mycoscience. 42 (1), 11-17 (2001).
- Idnurm, A., Walton, F. J., Floyd, A., Heitman, J. Identification of the sex genes in an early diverged fungus. Nature. 451 (7175), 193-196 (2008).
- Agrios, G. N. Plant pathogens and disease: general introduction. Encyclopedia of Microbiology. Schaecter, M. , Elsevier. 613-646 (2009).
- Valle, M., et al. Impact of water activity on the radial growth of fungi in a dairy environment. Food Res Int. 157, 111247(2022).
- Heo, Y. M., et al. Investigation of filamentous fungi producing safe, functional watersoluble pigments. Mycobiology. 46 (3), 269-277 (2018).
- Wickham, H. ggplot2 elegant graphics for data analysis. , Springer International Publishing. Cham, Switzerland. (2016).
- Lubkowitz, M. A., et al. Schizosaccharomyces pombe isp4 encodes a transporter representing a novel family of oligopeptide transporters. Mol Microbiol. 28 (4), 729-741 (1998).
- Nelson, M. A., Metzenberg, R. L. Sexual development genes of Neurospora crassa. Genetics. 132 (1), 149-162 (1992).
- Sato, S., Suzuki, H., Widyastuti, U., Hotta, Y., Tabata, S. Identification and characterization of genes induced during sexual differentiation in Schizosaccharomyces pombe. Curr Genet. 26 (1), 31-37 (1994).
- Grishkan, I., Korol, A. B., Nevo, E., Wasser, S. P. Ecological stress and sex evolution in soil microfungi. Proc Biol Sci. 270 (1510), 13-18 (2003).
- Bernstein, H., Byers, G. S., Michod, R. E. Evolution of sexual reproduction: importance of DNA repair, complementation, and variation. Am Nat. 117 (4), 537-549 (1981).
- Heitman, J., et al. The fungal kingdom. , ASM Press. (2017).
- Heitman, J., et al. Sex in fungi molecular determination and evolutionary implications. , ASM Press. (2007).
- Wang, Z., LópezGiráldez, F., Wang, J., Trail, F., Townsend, J. P. Integrative activity of mating loci, environmentally responsive genes, and secondary metabolism pathways during sexual development of Chaetomium globosum. mBio. 10, (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved