Method Article
Seizure Activity Induced by Electroshock in Drosophila Larvae
In This Article
Summary
This protocol details the use of Drosophila larvae to identify unique antiseizure compounds for the treatment of epilepsy.
Abstract
Epilepsy presents a significant health burden that is exacerbated by high numbers of individuals who are drug-refractory. Whilst some drug-refractory patients do respond to non-drug treatments (e.g., vagal nerve stimulation, ketogenic diet, etc.), the last resort for many is challenging and expensive surgery to provide relief from seizures. Whilst it is generally acknowledged that antiseizure medications with a broader range of targets are required, the hurdle in achieving this is the identification of novel drug targets. Genetically tractable model animals offer promise in this regard. The fruit fly, Drosophila melanogaster, has become a powerful model for investigating the mechanistic basis of, and better treatments for, seizures. Many identified fly mutations result in larvae and adults exhibiting seizure-like activity in response to strong stimulation (electrical, mechanical, and/or thermal). Many of these mutations are in genes homologous to those that contribute to human genetic epilepsies (e.g., the voltage-gated Na+ channel). It is also now possible to replace a fly gene with its human equivalent that additionally, carries a disease-related mutation. Thus, the humble fly has become an avatar to model human disease. This study describes a suitable method to use Drosophila larvae for low to medium-throughput drug screens to identify unique compounds, and their targets, that have antiseizure potential.
Introduction
Epilepsy remains a significant health burden, affecting approximately 1% of the population worldwide. Even though over 30 antiseizure medications (ASMs) now exist for clinical treatment, about one-third of people with epilepsy remain drug-refractory, meaning that they do not respond well to drug treatment1,2. Available drugs are also only palliative and, as such, do not prevent epileptogenesis, nor provide a cure3. Thus, there is a critical need to identify better epilepsy treatments. A roadblock to the development of more efficacious treatments is the identification of novel drug targets. Indeed, almost all current ASMs affect similar targets: ion channels, including the voltage-gated sodium channel (Nav), and inhibitory neurotransmission mediated by γ-aminobutyric acid (GABA)4,5. It is generally accepted that continued use of traditional methods of drug development is unlikely to radically change this scenario.
Laboratory model animals, including, but not limited to, the fruit fly Drosophila melanogaster, and the zebrafish Danio rerio, have utility for the identification of novel ASMs6,7,8. Indeed, a PubMed search for 'Drosophila + seizure' returns 342 results, whilst the same search for zebrafish returns 578 results (both searches were conducted on 29th Jan 2025). Whilst dwarfed by the number of similar studies in mice (~15,000), the number of studies using model systems continues to grow. These studies are possible due to the mechanistic conservation of CNS function across phyla. Moreover, induced seizures in flies and fish are effectively treated with clinically used ASMs, showing that whilst the nuances of seizure behavior may appear outwardly different, the underlying mechanisms have much in common7,9,10.
The fruit fly, Drosophila, has made many seminal contributions to understanding human biology. With respect to epilepsy, this model system provides an unparalleled genetic toolbox combined with identifiable and experimentally accessible neurons7. Moreover, the connectomes for both the larval and adult CNS have now been published, and numerous cell-specific genetic driver lines have been identified11,12. Significantly, a class of mutation was serendipitously identified whereby adult flies respond to strong mechanical stimulation by a loss of posture and seizure-like activity (e.g., wing buzzing, leg shaking, etc). This class of mutation has been termed 'bang-sensitive'13,14,15,16. A second class of seizure mutation has since been identified that responds to increased temperature, mirroring human febrile seizures17,18. However, the experimental tractability of adult flies is somewhat reduced compared to the larval stage of this same insect model. For example, it can be difficult to drug-feed adult flies, and more invasive techniques such as electrophysiology and optogenetics can be more challenging. By contrast, the Drosophila larva eats constantly to expand its body volume by ~100-fold in just 5 days to enable pupation. Therefore, we can be confident of adequate drug-feeding in larval stages. Embryogenesis is well documented and can be accurately staged, and it has, in turn, identified key milestones in CNS development, including the first acquisition of neuronal electrical properties through to circuit formation19. Once hatched, a larva goes through 3 molts (or instars) until, on day 5, it becomes 'wandering', whereupon it leaves the food to find a safe place to pupate. After ~100 h of pupation, an adult fly emerges with a new body and CNS (Figure 1).
Techniques to induce seizure in adults are not well suited to larval stages. Larvae lack sensory hairs, the synchronized activation of which during mechanical stimulation can lead to a seizure. Thus, to overcome these difficulties, an electroshock technique was developed to induce seizures at the wandering larval stage. The subsequent comparative analysis of seizure induction techniques across both larvae and adults reveals that larval electroshock is far less dependent on mutation type (e.g., bang-sensitive vs. temperature). Thus, we suggest that this technique should be the preferred method for testing novel mutations where the optimal seizure induction method is unknown20. The larval electroshock technique is simple, rapid, and requires minimal equipment. This technique provides an efficient means to screen novel compounds, or genetic therapies, for antiseizure efficacy across a range of mutations that mirror the genetic diversity of human epilepsy.
Protocol
The fruit fly, Drosophila melanogaster, is used in this study (see Results section for details). This technique is best suited to wandering third-instar larvae (L3). The experimental protocol is relatively simple but requires practice to perfect. In our experience, new students require about 2 weeks to master the assay and benefit greatly from viewing other, more experienced investigators perform the assay in real-time using a camera-enabled dissection microscope or similar. The reagents and the equipment used in this study are listed in the Table of Materials.
1. Larval selection
- Collect larvae from the sides of fly vials/bottles containing standard food (5 L of water, 390 g of glucose, 360 g of maize, 250 g of yeast, 40 g of agar, 135 mL of nipagin, 15 mL of propionic acid).
- Only use larvae that are actively moving and have left the food to crawl up the sides of the container (wandering third instars, L3). Do not select pre-pupae, which exhibit greatly reduced locomotion.
2. Electroshock procedure
- Remove a single wandering L3 and transfer it to a small plastic Petri dish (size does not matter), and gently wash it with ddH2O to remove food residue. A small (000) paintbrush is suitable for this.
- Transfer the single-washed larva, using a paintbrush, to an empty plastic dish (again, size does not matter). Dry the larva with a small fragment of paper towel held with forceps. Remove excess ddH2O, but do not completely dry the larvae to avoid it sticking to the plastic dish.
- Allow the larva to recover for 30 s. This will facilitate easy placement of the electroshock probe (see below).
- View the larvae under a low-power dissection microscope (15-20x), and once normal crawling behavior resumes, gently place the electroshock probe (see step 3 for details and Figure 2A) on the anterior dorsal surface of the larva above the approximate position of the CNS (see Figure 2C).
NOTE: This step is critical, enough pressure must be applied to provide good conductivity between probe wire and cuticle but take care not to damage the larvae. Thus, squash the larva by about one-third to one-half of its depth. - Apply a 2 s pulse of constant voltage, the strength of which has been pre-determined via a titration curve (Figure 3). Any isolated voltage stimulator is suitable here. The one used here is shown in Figure 2B.
- Following the electric shock, start a timer. In response to the shock, larvae initiate transitory paralysis followed by occasional spasms of body wall muscle activity and rolling behavior, halting normal crawling behavior.
- Stop the timer when the larva has clearly moved away from its original placement on the dish. Seizure duration, or recovery time (RT), is defined as the period between stimulus onset and resumption of normal crawling behavior (e.g., a full forward peristaltic wave that results in forward movement).
- At the end of each day, carefully clean the probe wires by first rinsing them in 100% ethanol followed by ddH2O. Carefully inspect the wires under magnification and, if required, use forceps to gently scrape any residue from the wires. Be very careful not to alter the distance between the two wires when doing so.
3. Electroshock probe contruction
- 2 x 1 m lengths of electrical wire (should be thin and flexible, i.e., rated for ~3 A) will be required. To the end of each wire, solder a 5 cm length of tungsten wire (to maximize contact, wind the tungsten wire around an exposed end of the electrical wire prior to soldering). Solder push-fit connectors (e.g., banana plugs) to the other end of the wires, suitable for easy connection to the voltage stimulator.
- Secure both tungsten wires to an electrode holder such that the wires are parallel to one another (Figure 2). Small sections of glass capillaries (~2 cm in length) are used to hold the wires in place under the probe lock screw.
- Use forceps to bend the wires such that they come to within 1-2 mm close to where they exit the wire holder. Bending tungsten wires is not easy because the wires retain a 'memory' - thus, perseverance is required. Tips to help this process include using electrical insulating tape and/or blu-tack (or similar modeling putty) to help maintain the wires in the correct orientation/distance.
4. Probe calibration
- Ensure that there are stocks of wandering L3 of a suitable wild type (negative control) and a seizure mutant (positive control). ~100 of each will be needed.
- Prepare larvae, one at a time, as described above.
- Use the probe to apply a range of voltages to sufficient larvae of each genotype for each voltage tested. It is suggested that 0 V, 2 V, 4 V, 6 V, 8 V, 10 V, and 12 V be applied for 2 s between 10-15 larvae per voltage. Shock each larva only once.
- Measure the recovery time for each larva and calculate the average for each voltage step applied for both genotypes.
- Plot the averages on a graph and fit the data with a straight line.
- Select a voltage with a clear and significant difference between the control and seizure mutant. Be careful not to choose a voltage that produces an overly long recovery time; otherwise, productivity will suffer because of the long wait time for recovery.
NOTE: This study often used a stimulation voltage that results in a recovery time of 50-100 s for wild type and 200-300 s for parabss. Exemplar calibration curves are shown in Figure 3. It is important to use 0 V to account for the act of pressing the probe onto the dorsal surface of a larva. This will cause some degree of paralysis, which is likely a defense mechanism of the larva.
5. Execution of experiments
- Electroshock larva of the desired genotype(s) or drug exposure and measure seizure recovery time.
NOTE: For test larvae, an n = 20 is usually sufficient, but a power calculation based on a pilot analysis will provide a more definitive n number. - Always run a negative (e.g., a wild type) and a positive (e.g., parabss) control during each experiment. The n numbers do not need to be high; n = 5 is sufficient. This will provide confidence that the assay has worked as expected (e.g., no issues with the probe or stimulator).
- Apply a cut-off (e.g., 300 s) to avoid overly long recovery times and only consider quantifiable seizures as those with recovery times above 30 s.
6. Drug screening
- Add drugs, dissolved in an appropriate solvent, directly to the food surface and allow to soak in (and if using ethanol, time for solvent evaporation). Or, alternatively, one can add a drug (in appropriate solvent) to melted fly food. See the Results section for details.
- To add the drug to the melted food, scoop out food from vials, re-melt, and as the food cools to 40 °C, add the drug, mix by vortex mixer, and then repour 5 mL of melted food back into vials and allow to cool before use.
- Run a concentration gradient to identify the optimal drug concentration.
NOTE: A good starting point is to add 3 mM drug solution either directly to the food surface (200 µL per standard Drosophila vial) or to make a 3 mM concentration in melted food. Adult females can be allowed to lay eggs directly in this food, or larvae can be added at selected stages as required. The solvent(s) used must be added alone to selected vials as vehicle control(s).
Results
Numerous Drosophila mutations exhibit enhanced seizure-like behavior7,20. The genetic basis of these mutations is varied, which favorably mimics the similarly varied genetic causes of human epilepsy. Three of the most studied Drosophila mutations are parabangsenseless (parabss), julius seizure (jus), and easily-shocked (eas). The parabss mutation results in a gain-of-function of the paralytic voltage-gated Na+ channel, eas encode ethanolamine kinase, and jus is an as yet unidentified membrane protein (note that jus was termed slamdance until recently)16,21,22. Many of these genes have human homologs. For example, paralytic is a homolog of human Nav genes, mutations of which are a leading cause of genetic epilepsy23. As with human epilepsy, these three 'bang-sensitive' fly mutations show differential seizure 'severity' in response to electric shock (Figure 4). The longest recovery time (i.e., most severe seizure behavior) is exhibited by parabss, whilst jus exhibits the shortest recovery time. This differential response, due to different underlying genetic mutations, allows an experimenter to test how lead compounds might reduce seizure across a range of mutations to identify those with favorable broad-spectrum activity.
Many ASMs used in the clinic are equally effective in Drosophila seizure models, hence indicating that the underlying mechanisms producing seizures in Drosophila are similar to those that result in seizures in people with epilepsy9,24,25. Figure 5 shows the effect of two of the most clinically-used ASMs, sodium valproate (VPA) and phenytoin (PHY), against parabss. Larvae were allowed to feed ad libitum on the ASM contained within food (added to melted food) at 3 mM during their entire larval development. Wild type (Canton-S, CS) and parabss fed vehicle control (ethanol and water, respectively) were included in each experiment, together with parabss fed the respective ASM. There is a clear and significant reduction in seizure recovery time (RT) for both drugs. Given that both wild type and a known seizure mutation (parabss), without exposure to ASM, exhibit expected seizure activity, we can be confident of the validity of these two experiments. When this is not the case, assays should be discarded, and the experiment should be repeated. The underlying cause for such 'failure' is often damage to the electroshock probe, low stimulator battery power, and/or experimenter inexperience. Maintenance of flies at temperatures below 25 °C should also be avoided (see Discussion section). Whilst this technique is not suited to high-throughput screening, recently, this was used in a low-throughput screen of ~30 compounds to identify a novel class of chemicals that effectively reduces seizure through manipulation of Pumilio - a regulator of neuronal homeostasis. A positive translation of these same compounds to reduce seizure in defined mouse seizure models shows an exciting potential for further development of both the lead compounds and the novel target26,27.
On examination of the representative results shown here (Figure 4 and Figure 5), it is evident that there is variability within the data spread. There is also a modest difference in the wildtype value between the data presented in these two figures. This is why this assay is qualitative and not suited to identify small differences in seizure severity, either between genotypes or in drug screening assays. However, the assay is well suited to identifying a seizure phenotype in unknown mutations and/or for initial testing of compounds for antiseizure activity27. Further refinements of drug development would, however, require different assay(s).
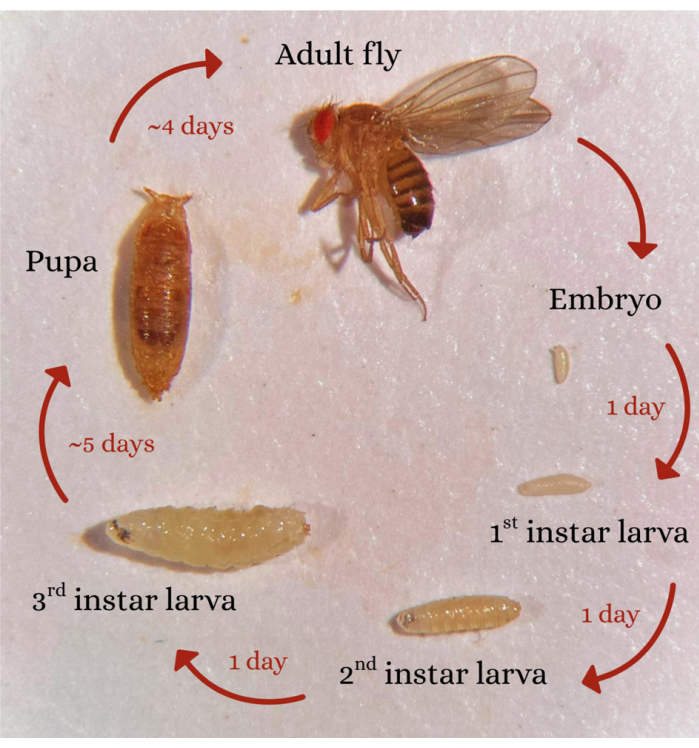
Figure 1: Drosophila lifecycle. A schematic showing the Drosophila life cycle, from embryo through to adult, via pupation. Timings shown are approximate for development at 25 °C. Please click here to view a larger version of this figure.
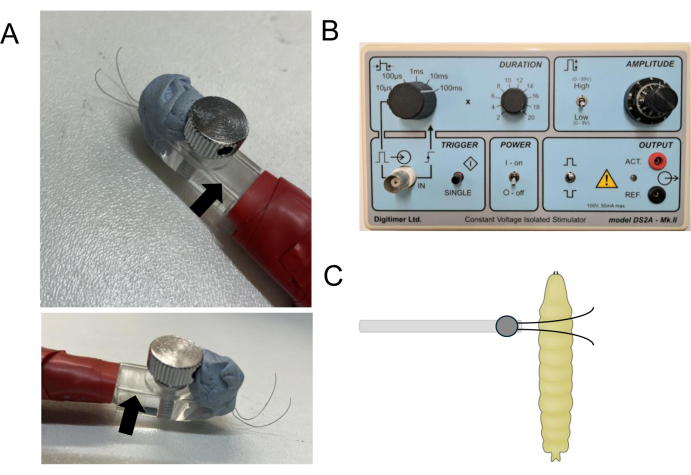
Figure 2: Design and placement of the electroshock probe. (A) shows images of a probe from both the top and side. Blu-tack has been used to maintain an inter-wire distance of ~2 mm as the tungsten wires extend from the probe. Short sections of glass capillaries (arrow) hold the tungsten wires in place. The electrical wires that connect to the stimulator are covered and held along the probe handle by electrical insulating tape. (B) shows a recommended constant voltage isolated stimulator used in this study. (C) shows the approximate positioning of the stimulating probe across the anterior dorsal surface of a wandering third-instar larva. Note the probe wires extend across the larval body, and the region immediately above the CNS is ~2 mm inter-wire distance. Please click here to view a larger version of this figure.
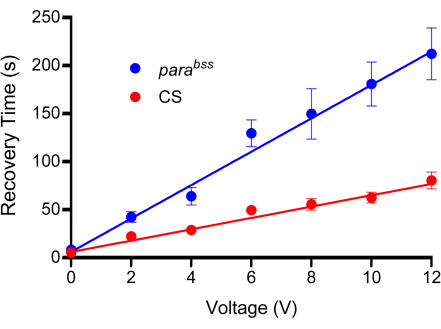
Figure 3: Calibration curve. Prior to experiments, a suitable voltage must be determined for electroshock. All probes will be slightly different due to the nature of their manufacture. A wild type (in this instance Canton-S, CS) and a seizure mutant (recommended, parabss) are subjected to increasing voltage shocks, each set at a fixed duration of 2 s. Larvae are only shocked once, and a minimum of n = 10 should be used for each voltage tested (in this case, n = 12). It is evident from the line fits that parabss show an increased seizure recovery time at all voltages. The voltage chosen should produce a clear and significant difference between controls and seizure mutants. In the plot shown, differences are significant at 6 V and above (p ≤ 0.0001, two-way ANOVA with Šidák's multiple comparisons, n = 12, per genotype and voltage). Thus, 6 V was chosen as the optimal voltage for this probe, as it produced a significant difference, yet the recovery time is kept short, reducing the time taken to conduct individual electric shocks. Data are presented as mean ± SEM. Please click here to view a larger version of this figure.
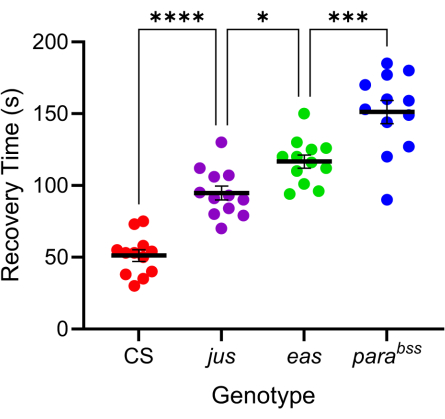
Figure 4: Different Drosophila mutations exhibit varying levels of seizure severity. Using the probe calibrated in Figure 3, three Drosophila seizure mutants (parabss, jus and eas), together with a wild type (CS), were subjected to electroshock (6 V, 2 s). Individual larvae were only shocked once. Seizure recovery times are greater for seizure mutants than wild type and increase progressively from jus to parabss(p ≤ 0.0001, respectively, one-way ANOVA with Tukey's multiple comparisons). Statistically significant comparisons from Tukey's multiple comparisons are indicated as ****p ≤ 0.0001, ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05. Data are presented as mean ± SEM. Please click here to view a larger version of this figure.
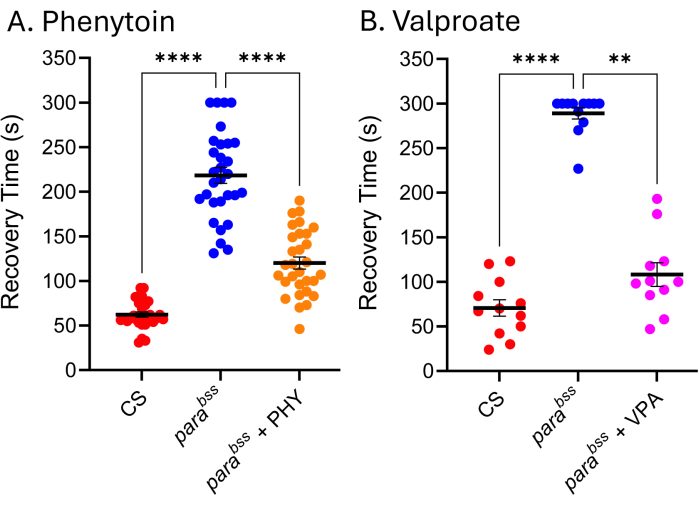
Figure 5: Drosophila seizure mutants respond to clinically used ASMs. Exposure to (A) phenytoin (PHY) and (B) sodium valproate (VPA) (3 mM, respectively) significantly reduced the seizure recovery time of parabss (p ≤ .01, Kruskal-Wallis with Dunn's multiple comparisons, and p ≤ .0001, one-way ANOVA with Tukey's multiple comparisons, respectively). Only recovery times above 30 s were considered a quantifiable seizure, and a cut-off of 300 s was used in these assays. Each drug assay was conducted by a different experimenter; thus, the average recovery times shown differ (particularly evident for parabss without a drug). This underscores the fact that this assay is qualitative, and thus, a wildtype and seizure mutant should be included in each assay. This can allow drug activity to be normalized between experiments if required. Statistically significant comparisons from Dunn's and Tukey's multiple comparisons are indicated as ****p ≤ 0.0001, ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05. Data are presented as mean ± SEM. Please click here to view a larger version of this figure.
Discussion
The electroshock method to induce seizure in Drosophila larvae provides a simple, yet efficient screen to identify novel antiseizure compounds or genetic manipulations. However, because this is a qualitative assay, its main limitation is that the method cannot readily identify small effect sizes. Nevertheless, the medium throughput it allows, lending itself to screen up to ~5 compounds per week, per investigator, provides a very powerful whole animal assay. The simplicity of the technique is also suited for undergraduate-level laboratory projects. Thus, 3-4 researchers can screen many compounds in a relatively short amount of time, and for a lot less expenditure than using an equivalent mouse seizure assay. Automation of the assay would further increase throughput. However, this study did not succeed in doing this. A lithium-containing agar with two electrodes embedded within the agar was attempted to shock multiple larvae simultaneously, but without success. Other methods, possibly based on optogenetics, might be more suited for automation28.
The simplicity of the assay, however, undermines the requirement to become proficient. The positioning of the probe and the pressure applied during the electroshock are critical steps. Consistency in both reduces variability between recovery times for shocked larvae. The other main issue is how to recognize the seizure endpoint, which makes this assay qualitative because it depends on when an individual investigator chooses to stop the clock. When several experimenters in the same lab conduct these experiments, it is worthwhile to spend time viewing the same larval shocks to agree on what a seizure endpoint is. Doing so greatly reduces inter-person variability. However, it is still evident from the exemplary results shown in this report that, whilst effects are constant, timings can vary between experimenters. It is also important to note that larval development at temperatures below 25 °C (e.g., 18 °C) reduces seizure severity due to a decrease in neural activity during an embryonic critical period29. Thus, it is suggested that a minimum of 25 °C is used for embryonic/larval development. Having an incubator close to the workbench is desirable, and only sufficient larvae are removed as required for each electroshock. Remaining larvae should be kept at 25 °C until needed. With practice, experimenters can shock up to 4 larvae at a time, spacing out shocks by 10 s intervals. This approach greatly speeds progress, and an n of 40 larvae (or more) can be achieved in one day. The humidity was not measured or controlled during this study. A standard 12:12 light-dark cycle was selected, but this can be varied as the experimenter wishes.
The manufacture of a probe is the most challenging part of this method. Thus, once made, great care should be taken not to damage it. Any damage that occurs to the electroshock probe will often entail a repair, and thus, the probe needs to be recalibrated to determine the optimal voltage. The voltage is entirely dependent on the distance between the two wires, and as such, no two probes are likely to be identical. It is advisable to recalibrate a probe every month to ensure the optimal voltage has not changed. The strength of the electroshock is dependent on both the voltage applied and the duration of the voltage. Thus, shorter durations with higher voltages will likely produce the same response as longer durations, using weaker voltage stimulation. However, this space has not been investigated. The preference for 2 s duration (in this study) is easily accommodated without undue movement of the larvae being shocked. This enables a comparatively lower voltage strength, which avoids damage to the larval cuticle. The effect of the stimulation will also depend on the current strength (amps), which we do not measure. However, different stimulators will produce different amperages, and thus, it was found that using a Grass S88 stimulator25, a voltage of 50 V/ 3 s was used, whilst utilizing the stimulator used here, 6-12 V / 2 s was used, depending on the probe being used. The actual voltage/duration used is not overly important as long as it is sufficient to induce a recovery time longer than a wildtype control and does not inflict damage to the larva. Because the act of just placing a probe onto a larva (using 0 V as a control) causes some degree of freezing/paralysis (indicative of an attempt to avoid predation), there are no stimulation criteria that will not induce some 'seizure-like' behavior.
The electroshock assay is suited to screening drugs for antiseizure activity. An issue is always solvent. It was found that ethanol or acetone are better solvents than DMSO. In the current experiments, DMSO is tolerated up to ~1%, beyond which unacceptable lethality was observed in developing larvae. Of the two options to expose larva to drugs, either adding drug (dissolved in a suitable solvent) to the top of a food vial or adding to melted fly food, it was found that the latter produces better results in terms of reduced variability. However, the difference is marginal, and the former method is quicker. It is recommended that experimenters compare both methods and choose the one that is best suited. Flies can be allowed to lay eggs on drug-containing food, such that the entirety of larval development is exposed to dug. Alternatively, larvae can be placed on drug-containing food at any desired stage to identify the critical timing of drug activity. Feeding drugs to gravid females is an effective method to expose developing embryos to drugs25.
An alternate and commonly used seizure assay is the use of zebrafish embryos exposed to the proconvulsant pentylenetetrazole to screen for ASMs30. In our opinion, the Drosophila larval assay has many advantages. These include (1) many compounds are difficult to dissolve in the artificial seawater that the fish swim in; (2) the zebrafish assay relies on reducing swim length, which can occur either from exposure to an ASM or, counterintuitively, a proconvulsant compound. This is because increased seizure activity in zebrafish also results in reduced swimming10. Thus, any compound hits must be followed up with a secondary screen, which often requires measurement of early-inducible neuronal genes such as c-fos; (3) fish can only be used without an animal license up to day 5 after hatching. Of course, zebrafish have a distinct advantage over insects in that their primary excitatory neurotransmitter is glutamate and not acetylcholine31. A combination of the two assays, beginning with Drosophila and followed by zebrafish, might provide a very powerful screen before fewer hit compounds are tested in mice.
To summarize, the Drosophila larval electroshock assay, coupled with the high conservation in genetics and physiology shared between flies and mammals, provides a very effective, rapid, and inexpensive screen to identify novel antiseizure therapies. Increased adoption of this insect for drug screening will also address the urgent need to reduce the number of higher-order animals used for medical research.
Disclosures
The authors declare no competing interests.
Acknowledgements
We thank the many Baines lab personnel who have jointly developed this technique over many years, and in particular, Richard Marley, who put in a good deal of effort to make this technique robust and reliable. We thank Anna Munro for drawing the larvae, which is shown in Figure 2. Work in the Baines lab that has contributed to developing this technique has been generously supported by BBSRC, MRC, and the Wellcome Trust. This work is currently supported by funding from a Wellcome Trust investigator award to R.A.B. (Grant 217099/Z/19/Z). The development of this technique also benefited from the Manchester Fly Facility, which was established through funds from the University and the Wellcome Trust (Grant 087742/Z/08/Z).
Materials
| Name | Company | Catalog Number | Comments |
| Electrode holder | World Precision Instruments | M3301 | |
| Glass capillaries | Harvard Instruments | GC100F-10 | |
| Tungsten wire (99.95%) | Goodfellow Cambridge, UK | 0.1 mm diameter | |
| Voltage stimulator | Digitimer Ltd, UK | DS2A mkII | Constant Voltage Isolated Stimulator |
References
- Fiest, K. M., et al. Prevalence and incidence of epilepsy: A systematic review and meta-analysis of international studies. Neurology. 88 (3), 296-303 (2017).
- Knezevic, C. E., Marzinke, M. A. Clinical use and monitoring of antiepileptic drugs. J Appl Lab Med. 3 (1), 115-127 (2018).
- Denton, A., et al. Definitions and risk factors for drug-resistant epilepsy in an adult cohort. Front Neurol. 12, 777888(2021).
- Meldrum, B. S., Rogawski, M. A. Molecular targets for antiepileptic drug development. Neurotherapeutics. 4 (1), 18-61 (2007).
- Sanchez, J. D., Gomez-Carpintero, J., Gonzalez, J. F., Menendez, J. C. Twenty-first century antiepileptic drugs: An overview of their targets and synthetic approaches. Eur J Med Chem. 272, 116476(2024).
- Cunliffe, V. T., et al. Epilepsy research methods update: Understanding the causes of epileptic seizures and identifying new treatments using non-mammalian model organisms. Seizure. 24, 44-51 (2015).
- Baines, R. A., Giachello, C. N. G., Lin, W. H. Drosophila. In Models of seizures and epilepsy. (2nd ed.). , 345-358 (2017).
- Burrows, D. R. W., et al. Imaging epilepsy in larval zebrafish. Eur J Paediatr Neurol. 24, 70-80 (2020).
- Reynolds, E. R., et al. Treatment with the antiepileptic drugs phenytoin and gabapentin ameliorates seizure and paralysis of Drosophila bang-sensitive mutants. J Neurobiol. 58 (4), 503-513 (2004).
- Baraban, S. C., Dinday, M. T., Hortopan, G. A. Drug screening in SCN1A zebrafish mutant identifies clemizole as a potential Dravet syndrome treatment. Nat Commun. 4, 2410(2013).
- Scheffer, L. K., et al. A connectome and analysis of the adult Drosophila central brain. Elife. 9, e57443(2020).
- Winding, M., et al. The connectome of an insect brain. Science. 379 (6636), eadd9330(2023).
- Ganetzky, B., Wu, C. F. Drosophila mutants with opposing effects on nerve excitability: Genetic and spatial interactions in repetitive firing. J Neurophysiol. 47 (3), 501-514 (1982).
- Song, J., Tanouye, M. A. From bench to drug: Human seizure modeling using Drosophila. Prog Neurobiol. 84 (2), 182-191 (2008).
- Parker, L., Howlett, I. C., Rusan, Z. M., Tanouye, M. A. Seizure and epilepsy: Studies of seizure disorders in Drosophila. Int Rev Neurobiol. 99, 1-21 (2011).
- Parker, L., Padilla, M., Du, Y., Dong, K., Tanouye, M. A. Drosophila as a model for epilepsy: BSS is a gain-of-function mutation in the para sodium channel gene that leads to seizures. Genetics. 187 (2), 523-534 (2011).
- Schutte, R. J., et al. Knock-in model of Dravet syndrome reveals a constitutive and conditional reduction in sodium current. J Neurophysiol. 112 (4), 903-912 (2014).
- Das, A., Smith, M. A., O'Dowd, D. K. A behavioral screen for heat-induced seizures in mouse models of epilepsy. J Vis Exp. (173), e62846(2021).
- Baines, R. A., Bate, M. Electrophysiological development of central neurons in the Drosophila embryo. J Neurosci. 18 (12), 4673-4683 (1998).
- Mituzaite, J., Petersen, R., Claridge-Chang, A., Baines, R. A. Characterization of seizure induction methods in Drosophila. eNeuro. 8 (4), (2021).
- Pavlidis, P., Ramaswami, M., Tanouye, M. A. The Drosophila easily shocked gene: A mutation in a phospholipid synthetic pathway causes seizure, neuronal failure, and paralysis. Cell. 79 (1), 23-33 (1994).
- Horne, M., et al. julius seizure, a Drosophila mutant, defines a neuronal population underlying epileptogenesis. Genetics. 205 (3), 1261-1269 (2017).
- Menezes, L. F. S., Sabia Junior, E. F., Tibery, D. V., Carneiro, L. D. A., Schwartz, E. F. Epilepsy-related voltage-gated sodium channelopathies: A review. Front Pharmacol. 11, 1276(2020).
- Kuebler, D., Tanouye, M. Anticonvulsant valproate reduces seizure-susceptibility in mutant Drosophila. Brain Res. 958 (1), 3642(2002).
- Marley, R., Baines, R. A. Increased persistent Na+ current contributes to seizure in the slamdance bang-sensitive Drosophila mutant. J Neurophysiol. 106 (1), 18-29 (2011).
- Lin, W. H., Giachello, C. N., Baines, R. A. Seizure control through genetic and pharmacological manipulation of Pumilio in Drosophila: A key component of neuronal homeostasis. Dis Model Mech. 10 (2), 141-150 (2017).
- Mulroe, F., et al. Targeting firing rate neuronal homeostasis can prevent seizures. Dis Model Mech. 15 (10), dmm049703(2022).
- Saras, A., Wu, V. V., Brawer, H. J., Tanouye, M. A. Investigation of seizure-susceptibility in a Drosophila melanogaster model of human epilepsy with optogenetic stimulation. Genetics. 206 (4), 1739-1746 (2017).
- Giachello, C. N., Baines, R. A. Inappropriate neural activity during a sensitive period in embryogenesis results in persistent seizure-like behavior. Curr Biol. 25, 1-5 (2015).
- Baraban, S. C., Taylor, M. R., Castro, P. A., Baier, H. Pentylenetetrazole-induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience. 131 (3), 759-768 (2005).
- Gauthier, M. State of the art on insect nicotinic acetylcholine receptor function in learning and memory. Adv Exp Med Biol. 683, 97-115 (2010).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved