Method Article
Arthroscopic Superior Capsule Reconstruction for Irreparable Posterosuperior Rotator Cuff Tears with Autologous Fascia Lata Graft
In This Article
Summary
This protocol outlines the steps for superior capsule reconstruction with fascia lata autograft, aiming to restore shoulder stability and function in patients with irreparable rotator cuff tears, followed by structured postoperative rehabilitation for optimal healing.
Abstract
This study explores the efficacy of superior capsule reconstruction (SCR) using fascia lata autograft for treating irreparable rotator cuff tears. Based on our experience and existing literature, SCR has demonstrated promising outcomes, offering improved shoulder stability, reduced pain, and prevention of humeral head migration. Patients undergoing SCR with fascia lata autograft achieved near-normal shoulder range of motion postoperatively, with maintained acromio-humeral distance observed in radiographic follow-ups. Various graft modifications, including the use of dermal grafts and the long head of the biceps tendon, have been explored. Yet, studies indicate the superior thickness and tensile strength of fascia lata autograft supports more durable outcomes. The SCR protocol detailed in this study includes meticulous graft harvesting, arthroscopic examination, and precise graft placement with suture anchors to ensure stability. Postoperative care involves immobilization followed by gradual rehabilitation, promoting effective healing and functional recovery. This approach highlights SCR's potential as a valuable treatment for active patients with irreparable rotator cuff tears.
Introduction
Despite advancements in medical science, a definitive gold standard for the treatment of massive irreparable rotator cuff tears has yet to be established. Historically, several treatment options have been attempted, including debridement, partial repair, and conservative management1,2,3,4. In recent years, numerous innovative surgical techniques have been introduced and have demonstrated favorable short-term outcomes3,5,6,7,8. One of the techniques developed is superior capsule reconstruction (SCR), which was introduced by Mihata et al. in 20139. The primary goal of SCR is to restore superior glenohumeral stability and shoulder function by preventing superior migration of the humeral head in patients with irreparable rotator cuff tears. Notably, SCR was originally developed in Japan, where reverse total shoulder arthroplasty (RTSA) had not yet been approved at the time. This historical context highlights that SCR was intended as a joint-preserving alternative for irreparable rotator cuff tears. It has been biomechanically proven to stabilize the glenohumeral joint and prevent proximal migration of the humeral head10. Currently, the most suitable indication for SCR is considered to be irreparable posterosuperior massive rotator cuff tears with Hamada stage 2 or less. Conversely, for irreparable posterosuperior massive rotator cuff tears with Hamada stage 3 or greater, or when accompanied by an irreparable subscapularis tear, SCR is considered contraindicated.
Recently, several modified SCR techniques have been developed, incorporating various approaches and different graft materials, including the use of the long head of the biceps tendon (LHBT) or dermal grafts11,12,13,14. However, biomechanical studies suggest that the traditional fascia lata autograft provides superior graft thickness, stiffness, and tensile strength compared to acellular dermal allografts15,16. In this protocol, we describe our approach using an autologous fascia lata graft to achieve SCR. This method is best suited for younger patients with irreparable posterosuperior rotator cuff tears with Hamada stage 2 or less, where joint-preserving treatment is prioritized over prosthetic replacement.
Protocol
This protocol was approved by the Institutional Review Board of the Chang Gung Medical Foundation (IRB No. 202000604B0), and informed consent was obtained from all participants.
1. Harvesting the Tensor Fascia Lata autograft
- Under general anesthesia, place the patient initially placed in the lateral decubitus position to facilitate graft harvesting.
- After standard sterile preparation of the skin with povidone-iodine solution and sterile draping of the operative field, make a longitudinal single incision approximately 8 cm in length over the proximal lateral thigh, centered over the tensor fascia lata, using a No. 10 scalpel blade. Perform careful dissection through the subcutaneous tissue and down to the fascia lata using Metzenbaum scissors and electrocautery, while ensuring meticulous hemostasis.
- Harvest a strip of the fascia lata measuring approximately 4 cm in width and 12 cm in length using a scalpel and Metzenbaum scissors. (Figure 1) Pay attention to maintain uniform thickness and avoid injury to the underlying muscle. Wrap the graft in moist gauze soaked in saline and set aside in a sterile fashion.
- After achieving hemostasis, irrigate the donor site with sterile saline solution. Close the deep fascia and subcutaneous layers using interrupted absorbable sutures with 1-0 Vicryl. Close the skin with either interrupted or subcuticular sutures using a 2-0 and 3-0 Vicry, followed by application of a sterile compressive dressing.
- Reposition the patient into the beach chair position in preparation for arthroscopic superior capsule reconstruction.
2. Arthroscopic examination
- Create five standard arthroscopic portals-posterior, anterior, lateral, anterolateral, and posterolateral-each made with a 5 mm skin incision using a No. 11 blade.
- Perform a systematic arthroscopic examination to assess intra-articular pathology. Begin with a standard posterior portal to evaluate the glenohumeral joint and establish an anterior portal under direct visualization as needed.
- Examine the articular cartilage of the humeral head and glenoid for chondral lesions or degeneration. Inspect the glenoid labrum circumferentially for any detachment, fraying, or tears, particularly in the superior and posterosuperior regions.
- Evaluate the joint capsule for any synovitis, redundancy, or scarring. Pay special attention to the long head of the biceps tendon (LHBT), including its origin at the superior labrum and its course through the bicipital groove. Assess for tendinopathy, subluxation, or SLAP (superior labrum anterior and posterior) lesions. (Figure 2A).
- In cases of articular-sided or upper full-thickness subscapularis tears, use a double-loaded suture anchor to reattach the tendon back to the footprint of the lesser tuberosity.
- Introduce arthroscopy into the subacromial space to check the posterosuperior massive cuff tear.
3. Placement of suture anchors at the glenoid and humeral sites
- Generally, the severely retracted cuff can be seen clearly through the posterolateral portal. Perform a thorough subacromial bursectomy and tendon stump debridement using a motorized shaver and radiofrequency device through the anterolateral and lateral working portals.
- Begin with bursectomy to improve visualization and working space in the subacromial space. Next, debride the rotator cuff tendon stumps to remove frayed or degenerated tendon tissue, creating a healthy bleeding surface to facilitate graft healing.
- Perform acromioplasty using a burr through the lateral portal to flatten the undersurface of the acromion. This step helps to increase the subacromial working space and reduce the risk of postoperative graft impingement. Perform these preparatory procedures to ensure smooth graft passage and optimal visualization during graft fixation.
- Create a bleeding surface by debridement over the superior glenoid. Place two 2.3 mm all-suture anchors at the anterosuperior and posterosuperior margins of the glenoid. A drill guide is inserted through the working portal, and a dedicated drill is used to create pilot holes. The anchors are then inserted and deployed according to the manufacturer's technique to ensure secure fixation at the glenoid rim. (Figure 2B,C).
- Decorticate and expose the greater tuberosity footprint. Insert two additional all-suture anchors at the cartilage margin of the supraspinatus footprint to ensure full defect coverage (Figure 2D).
- Retrieve all the sutures to the lateral portal to facilitate their subsequent passage through the graft.
4. Preparation of graft
- Fold the fascia lata autograft into a patch measuring 3 cm in width and 4 cm in length. Ensure the graft thickness is between 6 mm and 8 mm (Figure 3A,B).
- Place multiple Krackow and mattress sutures along the medial and lateral edges of the graft using No. 2 absorbable braided sutures. This configuration helps prevent graft flipping during arthroscopic insertion and allows for better control of graft orientation during fixation.
- Extend the lateral portal by 2 mm, both superiorly and inferiorly, to facilitate smoother graft passage (Figure 3C).
5. Insertion of graft into the subacromial space
- Pass the preloaded No. 2 high-strength nonabsorbable sutures from the two all-suture anchors on the glenoid side and the two on the humeral side through the corresponding medial and lateral edges of the graft, respectively. Ensure all sutures are passed at least 5 mm from the edge of the graft (Figure 3C).
- Insert the graft through the lateral portal in a controlled manner. To prevent suture entanglement, use a knot pusher to sequentially tense and guide each suture strand during graft advancement. This technique helps to maintain proper suture orientation and facilitates smooth graft delivery into the joint space.
- Tie the sutures with mattress configuration from the glenoid anchors upon confirming the proper positioning of the graft on the glenoid side. Then, tie the sutures with mattress configuration from the cartilage margin to secure the graft onto the supraspinatus footprint (Figure 3D).
- Insert two knotless anchors at the lateral edge of the tuberosity, utilizing a double-row technique to secure the graft in place (Figure 3E).
- Proper graft tensioning is critical to restoring superior glenohumeral stability in SCR. During graft fixation, maintain the glenohumeral joint in approximately 45° of abduction to optimize graft tension. This position is based on the biomechanical evidence reported where fixation done at 15° to 45° of arm abduction yields favorable outcomes for autologous fascia lata grafts17.
- Perform side-to-side suturing between the fascia lata autograft and the native infraspinatus tendon to enhance the posterior force couple. Use a curved suture hook to facilitate passage of sutures through both the thick autograft and the infraspinatus tendon.
- Place 1-2 interrupted stitches along the lateral edge to ensure robust soft tissue integration. Cove the entire exposed footprint by the graft-tendon complex (Figure 3F). Close the wounds with 3-0 Nylon sutures.
6. Postoperative care
- Put an abduction brace on the patient to immobilize the operative arm for a duration of 6 weeks. During this immobilization period, maintain the arm at 45° of abduction to support healing.
- After 6 weeks, commence rehabilitation with passive and active-assisted exercises, particularly focusing on scapular plane elevation, or scaption. Gradual progression from passive movements to more active-assisted exercises is key to avoiding early strain on the repair.
- By 8 weeks postoperatively, introduce strengthening exercises, targeting both the rotator cuff muscles and the scapular stabilizers.
- Initiate active motion around 12 weeks postoperatively, provided that patients have achieved near-complete passive range of motion. This progression ensures protection of the reconstruction while facilitating functional recovery.
Results
At 1 year postoperatively, patients who underwent SCR using the aforementioned technique demonstrated near-normal recovery of shoulder joint range of motion, including forward flexion, external rotation, and internal rotation (Figure 4A). Radiographic follow-up at the same time point showed that the acromiohumeral distance (AHD) was also well-maintained. (Figure 4B).
Based on our published series, patients undergoing SCR with fascia lata autograft showed significant improvements in shoulder function and joint stability after a minimum 2-year follow-up. Functional scores, including American Shoulder and Elbow Surgeons (ASES), the University of California-Los Angeles (UCLA), and Quick-DASH scores, were significantly improved, indicating a successful restoration of shoulder mobility and strength. Specifically, forward flexion increased from 75.6° to 157.2°, and external rotation improved from 33.3° to 53.3°, demonstrating substantial gains in range of motion. Radiographically, the AHD increased from an average of 6.1 mm preoperatively to 8.5 mm at follow-up, suggesting effective prevention of superior migration of the humeral head. The favorable clinical outcomes reinforce the feasibility of this approach in joint-preserving shoulder surgery18.
Mihata et al.'s 5-year follow-up on SCR also showed significant long-term improvements in function and pain relief. Shoulder function, as measured by the ASES and Japanese Orthopaedic Association (JOA) scores, increased substantially, with active elevation improving from 85° to 151°and external rotation from 27° to 41°. Pain levels, measured by the visual analog scale (VAS), decreased markedly from 6.9 to 0.9. Additionally, 92% of patients returned to work, and 100% resumed sports activities. Radiographic results indicated that SCR effectively maintained AHD, preventing humeral head migration and preserving joint stability. Graft integrity was key, as patients with intact grafts avoided the progression of cuff tear arthropathy, while those with graft tears developed severe arthropathy19. These findings support SCR as a durable, joint-preserving option for active patients with irreparable rotator cuff tears, enhancing function and reducing pain over the long term.
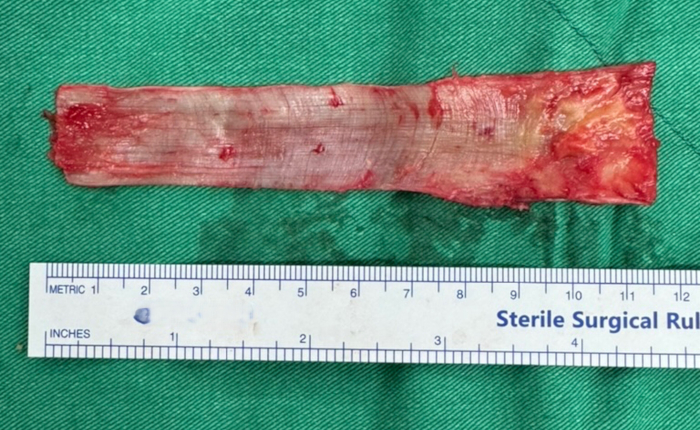
Figure 1: Harvested autologous tensor fascia lata graft. A tensor fascia lata graft measuring approximately 4 cm in width and 12 cm in length. Please click here to view a larger version of this figure.

Figure 2: Suture anchors at the glenoid and humeral sites. (A) Arthroscopic examination from the posterior portal; the subscapularis is repaired if necessary. (B, C) Viewing from the posterolateral portal, two suture anchors are placed at the posterosuperior and anterosuperior aspects of the glenoid. (D) After adequate decortication, two suture anchors were inserted at the cartilage margin of the supraspinatus footprint. Abbreviations: SSC = subscapularis; G = glenoid; H = humerus; FT = footprint of supraspinatus. Please click here to view a larger version of this figure.
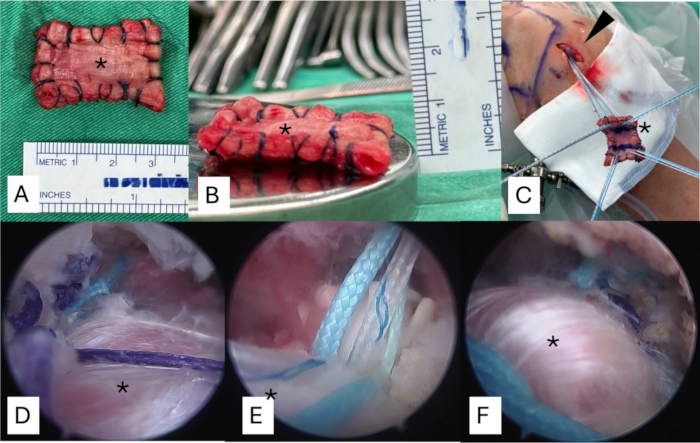
Figure 3: Preparation of fascia lata autograft to the appropriate thickness and shuttling the graft into the subacromial space. (A) The graft is folded into a patch measuring 3 cm in width and 4 cm in length. (B) The graft thickness should be between 6 mm and 8 mm. (C) The sutures from the anchors are passed through each side of the graft and then shuttled to the subacromial space from the lateral portal. (D) The sutures from the glenoid anchors are securely tied first after confirming the proper positioning of the graft on the glenoid side. (E) Two knotless anchors are placed at the lateral edge of tuberosity. (F) The graft is finally secured using a suture bridge technique, ensuring full coverage of the area of the massive tear. The asteroid shows the fascia lata autograft. Please click here to view a larger version of this figure.
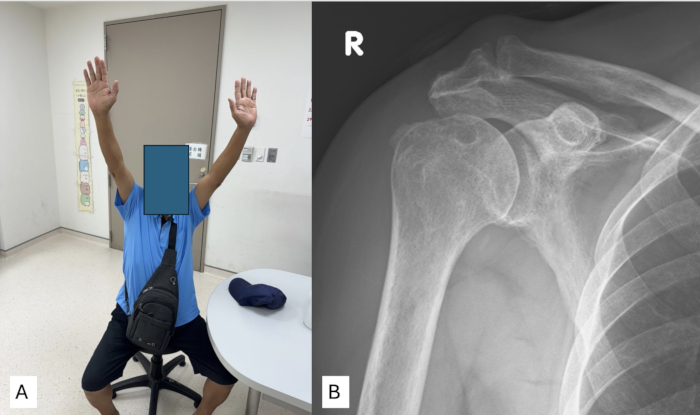
Figure 4: Postoperative outcomes for the patient who underwent superior capsule reconstruction using fascia lata autograft. (A) Physical examination after 1 year shows that the patient's active range of motion has nearly returned to normal levels. (B) Shoulder anteroposterior (AP) X-ray after 1 year demonstrates stable maintenance of the acromiohumeral distance after surgery. Please click here to view a larger version of this figure.
Discussion
Since its introduction by Mihata et al. in 2013, SCR has undergone various developments, particularly in selecting graft materials, which have seen significant variation. This is likely one of the reasons for the inconsistent clinical outcomes observed in previous literature regarding SCR20. Previous studies by Mihata have demonstrated that using a fascia lata autograft provides significant biomechanical benefits in preventing superior migration of the humeral head and reducing subacromial contact pressure10,16. Moreover, it has been shown that, in comparison to fascia lata autografts, using a single-layer dermal graft is less effective due to its inadequate thickness16. In other words, graft thickness is critically important, which is one of the key advantages of using the tensor fascia lata. Apart from thickness, the fascia lata is also shown to possess superior stiffness. In experiments by Mihata et al., it was found that dermal allografts used in SCR can elongate by up to 15% after only a few physiological shoulder movements, whereas fascia lata grafts do not exhibit this elongation21. This may also explain the frequent dermal graft failures reported in previous clinical outcomes12. Thus, we have described the surgical technique and approach using fascia lata graft to achieve favorable clinical outcomes in SCR. The most critical step in this procedure is obtaining a graft of sufficient thickness, and particular attention must be given to ensuring the graft has adequate length and width during the initial harvest of the fascia lata.
As previously mentioned, there have been numerous variations in SCR techniques in recent years11,13,18,22,23. In addition to the dermis graft, which has shown variable prognosis, the autologous LHBT is another widely accepted graft option. The use of the LHBT, also known as the Chinese way, has undergone various modifications since its introduction in 201711,22. These variations include different routing techniques, as well as combinations with fascia lata and dermis grafts13. The biceps tendon graft has also demonstrated promising outcomes; however, clinical data directly comparing it with the fascia lata graft remains limited, highlighting the need for further research.
Although using fascia lata for SCR is a well-established procedure and has demonstrated excellent mid- to long-term outcomes in reports by Mihata et al.9,19, it still presents certain limitations. First, the harvest of fascia lata can lead to donor site morbidity, including pain and complications at the harvest site. A study by Ângelo et al. evaluated this morbidity in 15 patients over a mean follow-up of 2.5 years. They reported that 20% of patients experienced mild donor-site pain, and 13.3% had mild thigh hypoesthesia. Importantly, no patients reported functional deficits or dissatisfaction related to the donor site24. Additionally, fascia lata autografts may vary in thickness and quality, potentially affecting graft performance. The quality of the graft may determine whether the outcomes of SCR surgery are reproducible. Furthermore, fascia lata requires more extensive preparation and surgical time compared to alternative grafts, such as dermal allografts. These factors contribute to its challenges in clinical practice. Thus, following an established protocol when using fascia lata to achieve favorable outcomes is essential, which is a key objective conveyed in this article.
Compared with traditional methods for treating massive irreparable rotator cuff tears, SCR stands out as a joint-preserving technique, providing patients with effective pain relief and restoration of range of motion. This method aims to restore glenohumeral stability by reconstructing the superior capsule, which in turn prevents superior migration of the humeral head, thereby further delaying the progression of cuff arthropathy. In the future, there may be more studies combining different surgical techniques, and comparing these various approaches will be an important direction for further research.
Disclosures
The authors report no conflicts of interest or financial disclosures related to this work.
Acknowledgements
The authors gratefully thank the Taiwan Minister of Science and Technology and Linkou Chang Gung Memorial Hospital for the financial support of this study (Grant: MOST 111-2628-B-182A-016, NSTC112-2628-B-182A-002, CMRPG5K0092, CMRPG3M2032, CMRPG5K021, SMRPG3N0011)
Materials
| Name | Company | Catalog Number | Comments |
| Footprint knotless PEEK suture anchor | Smith & Nephew, Andover, MA | Two 4.5 mm anchors for lateral row fixation over humeral site | |
| Glenoid and humerus anchors | Stryker | Iconix | All-suture anchor |
| Iconix suture anchor | Stryker Endoscopy, San Jose, CA | Two 2.3 mm anchors for glenoid site and two 2.3 mm anchors for medial row fixation over humeral site | |
| Lateral row anchor | Smith & Nephew | Footprint Ultra | For Future-bridge repair |
References
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved