Method Article
Novel Mini-open Transforaminal Lumbar Interbody Fusion
In This Article
Summary
The protocol introduces a novel mini-open TLIF, which can significantly reduce intraoperative blood loss, achieving minimally invasive outcomes with enhanced recovery.
Abstract
Transforaminal lumbar interbody fusion (TLIF) is an effective and popular surgical procedure for managing various spinal pathologies, particularly degenerative diseases. Since the advent of TLIF, surgeons have pursued minimally invasive techniques. Currently, TLIF can be performed through transforaminal approaches by open surgery, minimally invasive surgery, or percutaneous endoscopy. This study provides a detailed description of a modified open TLIF with percutaneous pedicle screws, referred to as mini-open TLIF. The objective is to present the feasibility of this procedure and its preliminary results. The procedure has been performed since January 2021, and the number of patients meeting the inclusion criteria has exceeded 300. Data collected include operation time, blood loss, ambulatory time, hematocrit levels, and perioperative complications. Clinical symptoms are evaluated at 1 week, 3 months, and 12 months postoperatively. Visual analog scale (VAS) scores for lower back and leg pain and the Oswestry Disability Index (ODI) are assessed. Magnetic resonance imaging is conducted preoperatively and 12 months postoperatively to measure the cross-sectional area of paraspinal muscles. Lumbar interbody fusion rates are evaluated using CT scans. The procedure can be applied to most common lumbar degenerative diseases in clinical practice. The currently collected data indicates that the mean operation time for single level was 102.3 min and 130.2 min for multi-level procedures. Intraoperative blood loss averaged 62.5 mL for single-level and 108.3 mL for multi-level surgeries. VAS and ODI scores showed significant improvements post-surgery (p < 0.001), achieving minimal clinically important differences. Paraspinal muscle atrophy rates were 2.5% on the symptomatic side and 1.2% on the asymptomatic side. Changes in cross-sectional areas and atrophy rates are not statistically significant (p > 0.05). MO-TLIF is effective and feasible for treating lumbar degenerative diseases, especially in multi-level cases, with minimal muscle damage and shorter operation times.
Introduction
Lumbar degenerative disease (LDD) is prevalent among the elderly population, often presenting with lumbar disc herniation and lumbar spinal stenosis, manifesting as chronic back pain and neurological symptoms1. Since its introduction in the 1980s, transforaminal lumbar interbody fusion (TLIF) has evolved and remains one of the most classic and well-established surgical procedures for treating lumbar degenerative diseases2. In 2002, Foley et al. introduced minimally invasive surgery transforaminal lumbar interbody fusion (MIS-TLIF)3,4,5. In 2012, Osman et al. reported using a single-channel endoscope on lumbar fusion6,7. In 2018, Kim and Choi introduced unilateral biportal endoscopy (UBE), successfully applying it to TLIF procedures, naming it biportal endoscopic TLIF (BE-TLIF)8.
The gradual adoption of MIS-TLIF and PE-TLIF in recent years has significantly improved clinical outcomes and patient satisfaction compared to traditional surgeries9. Some scholars even believe that endoscopic techniques will eventually replace open surgery. However, traditional open surgery is also continuously improving and becoming less invasive, with modified TLIF emerging as a widely accepted open procedure10. Modified TLIF involves incising the lumbodorsal fascia along the spinous process, meticulously detaching the muscles and ligaments attached to the spinous process and lamina under the periosteum and exposing the vertebral pedicles without extensive dissection or prolonged retraction. This technique achieves similar minimally invasive outcomes compared to the Wiltse approach11.
In this context, this study innovatively proposes a further advancement on minimally invasive approach, mini-open TLIF (MO-TLIF), assisted by percutaneous pedicle screws. Many studies report that posterior lumbar surgery often leads to postoperative paraspinal muscle atrophy, possibly related to prolonged retraction of back muscles during surgery, which is associated with low back pain (LBP) and radicular symptoms12,13,14. Mengiardi et al. found that increased fat infiltration in the multifidus muscle often results in chronic lower back pain7, while Hyun et al. suggested a link between lumbar radiculopathy and muscle denervation atrophy12. Other studies have shown that paraspinal muscle atrophy is closely related to the occurrence and aggravation of lumbar degenerative disease symptoms15. The quantity and function of paraspinal muscles play a crucial role in maintaining lumbar-pelvic sagittal balance and are essential for postoperative lumbar stability16. Therefore, the impact on paraspinal muscles is a critical consideration in selecting the surgical approach and technique for spinal fusion, with anterior or minimally invasive posterior approaches reducing muscle disruption17.
This study prospectively analyzes the short-term clinical efficacy and changes in paraspinal muscles in patients with single-level and multi-level lumbar degeneration treated with MO-TLIF (47 males and 49 females with an average age of 54.8 ± 17.5 years, as shown in Table 1). We evaluated surgical efficacy, blood loss, fusion outcomes, pain and functional scores, and paraspinal muscle disruption, comparing these outcomes with patients undergoing modified TLIF during the same period. This comparison aims to explore the advantages of MO-TLIF in treating lumbar degenerative diseases, particularly its impact on paraspinal muscles.
Protocol
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Second Affiliated Hospital of Soochow University (No. JD-LK2023045-I01). Informed consent was obtained from all individual participants included in the study. All images in the article obtained the informed consent of the human research participants.
1. Inclusion and exclusion criteria
- Use the following inclusion criteria: Clinically diagnosed with single-level or multi-level lumbar disc herniation, lumbar spinal stenosis, lumbar instability, or Grade I or II lumbar spondylolisthesis; persistent low back pain and/or leg pain, numbness, or weakness that has not responded to conservative treatment for 3 months.
- Use the following exclusion criteria: Revision surgery; presence of scoliosis, ankylosing spondylitis, new vertebral fractures, spinal tumors, or other malignancies; grade III or higher lumbar spondylolisthesis; patients with coagulation disorders.
2. Surgical procedure
- Preoperative preparation
- Position all patients who undergo surgery under general anesthesia prone on a radiolucent spinal surgery table. Position an appropriately sized support pad or cushion under the thoracic and pelvic area to elevate the chest and pelvis, respectively. Fine-tune the height and angle of the supports to maximize interbody exposure and allow the abdomen to droop freely to prevent inferior vena cava compression.
NOTE: Generally, an anterior lumbar lordosis of 10° to 15° and an appropriate pelvic tilt are considered optimal for achieving the best intervertebral space exposure. Fine adjustments during the operation are crucial to balance the desired exposure with patient safety. - Begin with the identification of the L5-S1 segment; utilize a localization guide under C-arm fluoroscopy on the basis of standard anteroposterior views to determine the lesion segment and the surface projection of the pedicles on the patient's body.
- Use a marker pen to mark midline surgical incisions, surface projections of pedicles, as well as 1 cm incisions at the lateral margin of pedicle projections.
- Disinfect the surgical site with iodophor in three separate applications, extending the disinfectant at least 15 cm beyond the planned incision to create an adequate antiseptic margin. Take care not to smear or remove the preoperative markings.
- Place four sterile drapes around the incision site. Apply surgical drapes at both the cephalad and caudal ends of the operative field. Cover the surgical area with a large sterile drape, securing it in place.
- Position all patients who undergo surgery under general anesthesia prone on a radiolucent spinal surgery table. Position an appropriately sized support pad or cushion under the thoracic and pelvic area to elevate the chest and pelvis, respectively. Fine-tune the height and angle of the supports to maximize interbody exposure and allow the abdomen to droop freely to prevent inferior vena cava compression.
- Establishment of the surgical access channel
- Make a 3 cm longitudinal incision along the marked line on the lower back with a #10-blade (Figure 1). Cut through the skin, subcutaneous tissue, and thoracolumbar fascia with high frequency electrotome in sequence.
- Detach the paraspinal muscles along the spinous process to expose the affected spinous process, lamina, and part of the facet joint with high frequency electrotome. Place the lamina retractor at the outer edge of the upper facet of the lower vertebra to expose the surgical field, typically establishing the approach channel within 5 min.
- Decompression
- Use an ultrasonic bone knife or ordinary bone knife to remove the superior subarticular process and part of the inferior supraspinous process. Remove part of the ventral ligamentum flavum to expose the dura mater and nerve roots. Meanwhile, preserve the dorsal ligamentum flavum and epidural fat to minimize disruption to the normal spinal canal anatomy.
- If bilateral decompression or contralateral stenosis is required, tilt the radiolucent spinal surgery table towards the contralateral side. Remove the base of the spinous process and resect the hypertrophic ligamentum flavum until the contralateral lateral recess is reached. This approach achieves a thorough 270° decompression.
- Retract the nerve roots and dural sac with a nerve root retractor to expose the operating area. Incise the annulus fibrosus with a #11-scalpel. Remove the nucleus pulposus with a Kerrison and scrape the endplate cartilage with a bone rongeur to expose the bony endplate. Sequentially dilate the intervertebral space with an intervertebral disc chisel and flush with normal saline to achieve hemostasis.
- Trim the excised articular processes and a portion of the lamina using a bone rongeur to create bone fragments approximately 2 mm2 in size. Pack a portion of these bone grafts into the cage and place the remaining fragments into the intervertebral space. Position the cage centrally within the intervertebral space.
- Use an ultrasonic bone knife or ordinary bone knife to remove the superior subarticular process and part of the inferior supraspinous process. Remove part of the ventral ligamentum flavum to expose the dura mater and nerve roots. Meanwhile, preserve the dorsal ligamentum flavum and epidural fat to minimize disruption to the normal spinal canal anatomy.
- Closing the incision
- Confirm the position of the intervertebral fusion device is satisfactory by lateral and anterio-posterior fluoroscopy. Use a neural stripper to probe the dural sac and nerve roots for good mobility, no compression, and no stenosis of the spinal canal.
- Flush the intervertebral space with saline solution. Use 3-0 absorbable sutures to close the fascia layer with a locking technique and perform continuous suturing for the fat layer. Suture the skin using either staples or sutures, depending on the preference and clinical requirements. This procedure does not require routine placement of drainage.
- Internal fixation placement and closed incision
- Make a 1 cm incision at the projection sites of the pedicles of the vertebrae above and below the targeted intervertebral space (the markers have been made preoperatively).
- Perform the following procedures under C-arm fluoroscopy to avoid violations of the pedicle or damage to surrounding nerves. Insert a sharp trocar needle through the skin to access the pedicle. Ensure precise positioning of the needle at the planned entry point.
- After correct needle placement, use a small-diameter reamer to gradually enlarge the pedicular channel, ensuring sufficient space for the pedicle screw. Utilize a dedicated guiding system to insert the pedicle screw and connecting rods into the reamed channel and then tighten the screw caps. Irrigate the incision with saline to achieve hemostasis thoroughly.
- Use 3-0 absorbable sutures to close the incision layer by layer and cover with a dressing. Check the lower limb activity postoperatively.
NOTE: For multi-level surgeries, the posterior midline incision is approximately 4 cm for two segments and 5 cm for three segments (Figure 2). The surgical approach channel can be established using the same method, only changing the position of the lamina retractor to decompress multiple intervertebral spaces. Slightly extending the multi-level incision can increase the operating space, making the procedure more convenient and providing better exposure without significantly increasing trauma and bleeding.
3. Clinical assessment
- Gather the following data preoperatively, intraoperative, 1 week postoperatively, 3 months postoperatively and 12 months postoperatively. Record operation time, blood loss, postoperative ambulatory time, follow-up time, complications, and visual analog scale (VAS) and Oswestry disability index (ODI) scores, which are extensively utilized for lumbar and lower extremity pain18.
- Measure the visible blood loss during surgery using a graduated suction bag. Subtract the volume of fluids, such as saline, used during the procedure. Additionally, estimate the blood volume absorbed by the gauze by weighing the blood-soaked gauze.
- Use preoperative and postoperative hematocrit (Hct) to calculate blood loss. Determine the patient's estimated blood volume (EBV) using the Nadler formula19:
EBV (mL)= [k1 x height (m)3 + k2 x weight (kg) + k3] x 1000]
For males: k1=0.3669, k2=0.03219, k3=0.6041. For females: k1=0.3561, k2=0.03308, k3=0.1833. - Subsequently, calculate the total blood loss (TBL) using the Gross formula20
TBL (mL)=EBV (mL) x (HctPre−HctPost)/HctAve
where HctPre represents the preoperative hematocrit, HctPost is the hematocrit value measured on the second postoperative day, and HctAve is the average of HctPre and HctPost. - Use lateral radiography and computed tomography (CT) to evaluate intervertebral fusion21. Perform magnetic resonance imaging (MRI) preoperatively and 1 year postoperatively to emulate cross-sectional area (CSA) of lesion segments' paraspinal muscles. Calculate properties of fat infiltration by ImageJ22 (Figure 3).
4. Statistical analysis
- Compare VAS and ODI scores before and after surgery by a paired-sample t-test. Compare the cross-sectional area measured with MRI T2WI at operational segments before and after operation. All statistical analyses were performed using SPSS. The data is presented as mean ± standard deviation, and p < 0.05 was considered significant.
Results
For single-level operation (n = 50), the mean operation time was 102.3 min (range 75-160 min) and 130.2 min (range 112-185 min) for multi-level surgeries (n = 46). The mean intraoperative blood loss for the single-level procedure was 62.5 mL (range 35-125 mL), and for the multi-level procedure, it was 108.3 mL (Table 2). The preoperative and postoperative cross-sectional areas (CSA) and fat infiltration (FI) levels of the bilateral paraspinal muscles are shown in Table 3.
Preoperatively, the CSA on the decompression side was 2088.4 ± 226.7 mm2, and on the contralateral side, it was 2081.8 ± 238.6 mm2, showing no significant difference. At 1 year postoperatively, the CSA on the decompression side was 2077.9 ± 225.5 mm2, and on the contralateral side, it was 2076.1 ± 235.5 mm2. The atrophy rate on the decompression side was 2.5%, while it was 1.2% on the contralateral side, with no statistically significant difference (p > 0.05).
The fat infiltration ratio on the decompression side was 22.14% ± 9.21% preoperatively and 22.09% ± 9.04% postoperatively. On the contralateral side, the fat infiltration ratio was 21.78% ± 8.71% preoperatively and 22.20% ± 9.19% postoperatively. There were no statistically significant differences in CSA comparison either preoperatively and postoperatively on the same side or between the decompression side and the contralateral side. Detailed data on paraspinal muscle disruption are provided in Table 3 and Table 4.
These results indicate that the intraoperative blood loss in MO-TLIF is lower than that of traditional open TLIF while being comparable to that of BE-TLIF. The surgical duration is significantly shorter than that of BE-TLIF yet similar to that of traditional open TLIF. Furthermore, based on the successful completion of over 400 procedures to date, MO-TLIF significantly reduced muscle invasiveness, demonstrating barely an increase in muscle damage on the decompression side compared to the contralateral side.
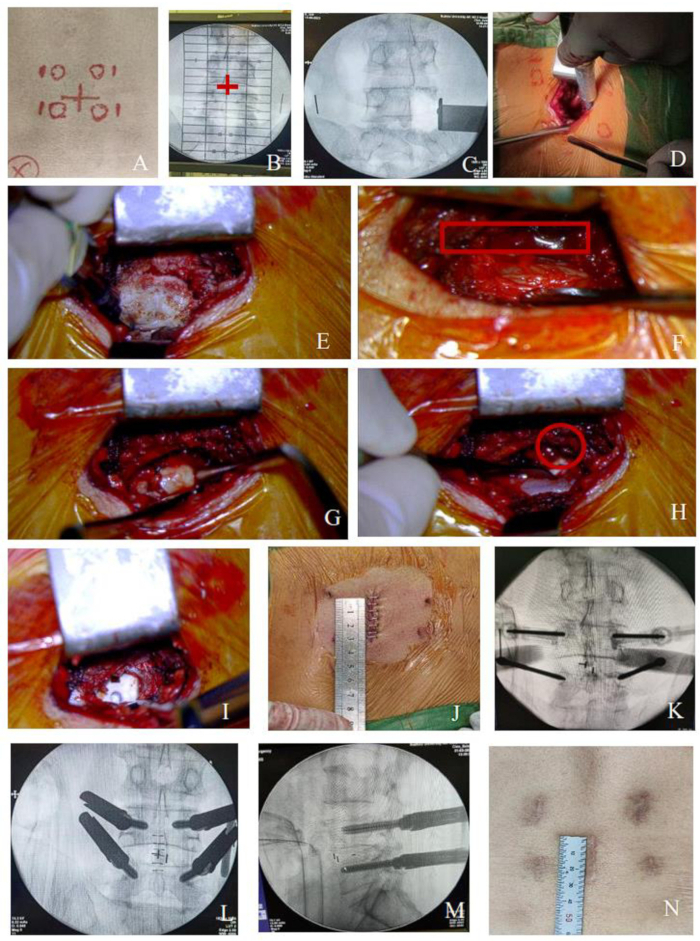
Figure 1: Procedure of MO-TLIF for the single- level lesion. (A-B) The intervertebral space and projection of the pedicle were determined under C-arm fluoroscopy, as was shown by the red cross. Then, mark the surgical incision along the spinous process between the two intervertebral spaces and 1.5 cm lateral to the pedicle mark the puncture points of Percutaneous pedicle screws. (C-I) Schematic diagrams of the surgical field during the decompression process (nerve root was encircled by a red solid line). (J) Central 3 cm incision for single-level lesion. (K-M) Process of percutaneous screw placement. (N) The incision after healing. Please click here to view a larger version of this figure.
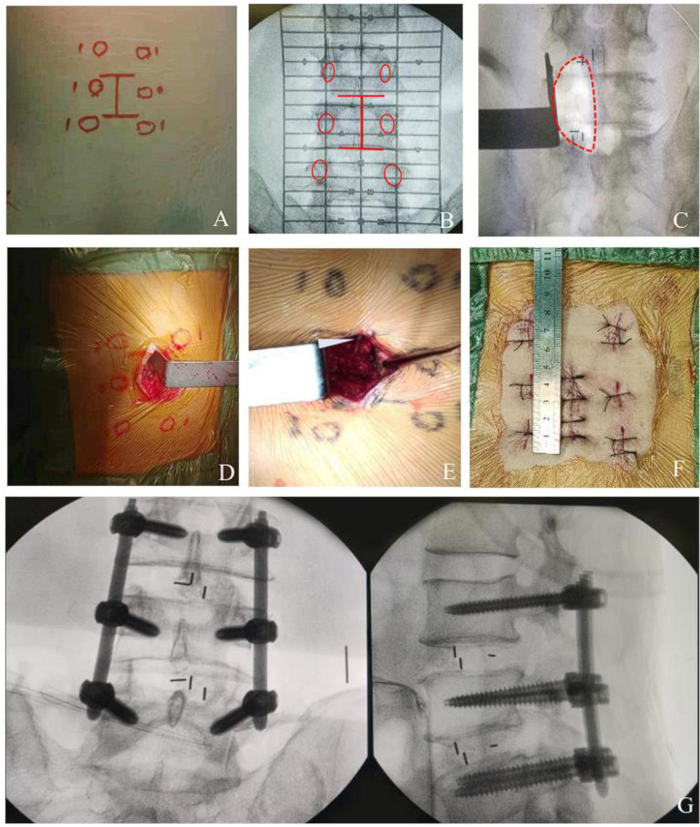
Figure 2: Procedure of MO-TLIF for multi- level lesion. (A-B) Multi-level mark line on skin. (C-E) surgical field of multi-level decompression. (F) Postoperative photograph showing the incision in MO-TLIF approximately 4 cm for two-segment surgery. (G) Photograph as MO-TLIF completed showing pedicle screws and cages in place. Please click here to view a larger version of this figure.
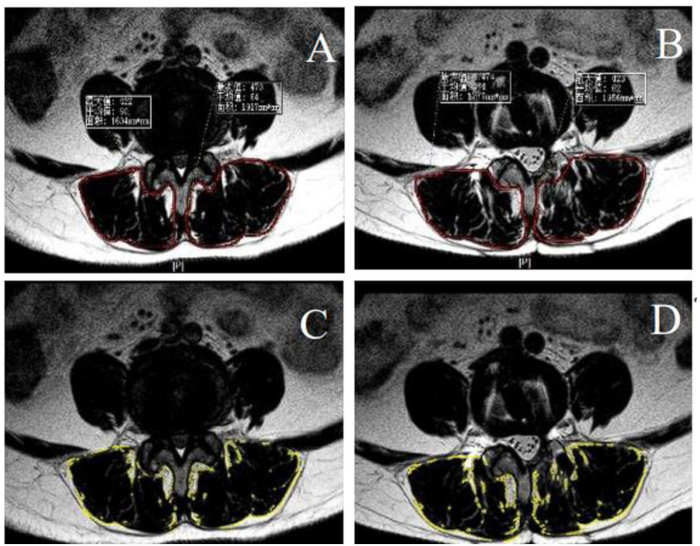
Figure 3: Preoperative and postoperative fat infiltration and cross-sectional area of paraspinal muscle. (A-B) Preoperative and postoperative cross-sectional areas of paraspinal muscle encircled by the red line. (C-D) Preoperative and postoperative fat infiltration of paraspinal muscle, Calculating the properties by ImageJ, were encircled by the yellow line. Please click here to view a larger version of this figure.
| Characteristics | Values |
| Mean age (years) | 54.8 ± 17.5 |
| Sex (M/F) | 47/49 |
| Diagnosis | |
| Lumbar spondylolisthesis | 21 |
| Lumbar disc herniation with segmental instability | 45 |
| Lumbar foraminal stenosis with segmental instability | 30 |
| BMI | 23.9 ± 2.8 |
| Operation Level | |
| Single-level | |
| L3-4 | 5 |
| L4-5 | 24 |
| L5-S1 | 21 |
| Multi-level | |
| L3-5 | 18 |
| L4-S1 | 24 |
| L3-S1 | 4 |
| Follow-up period (months) | 13.2 ± 2.1 |
Table 1: Demographic characteristics of patients.
| Metrics | Single-Level | Multi-level |
| Preoperative Hematocrit (%) | 41.6 ± 4.8 | 42.7 ± 7.1 |
| Postoperative Hematocrit (%) | 38.5 ± 3.4 | 38.8 ± 6.8 |
| Intraoperative Blood Loss (mL) | 62.5 ± 28.2 | 108.3 ± 31.2 |
| Estimated Total Blood Loss (mL) | 213.9 ± 124.8 | 282.8 ± 155.9 |
| Operation Time (min) | 102.3 ± 17.2 | 130.2 ± 18.3 |
| Postoperative Ambulation Time (days) | 1.7 ± 0.4 | 2.0 ± 0.5 |
Table 2: Perioperative data.
| Side | CSA (mm2) | FI (%) |
| Preoperative | ||
| Decompression Side | 2088.4 ± 226.7 | 22.14 ± 9.21 |
| Contralateral Side | 2081.8 ± 238.6 | 22.09 ± 9.04 |
| Postoperative | ||
| Decompression Side | 2077.9 ± 225.5 | 21.78 ± 8.71 |
| Contralateral Side | 2076.1 ± 235.5 | 22.20 ± 9.19 |
Table 3: Preoperative and postoperative paraspinal muscle CSA and fat infiltration.
| Mean ± SD (mm2) | p Value | |
| Preoperative Decompression Side - Preoperative Contralateral Side | 6.59 ± 36.65 | 0.081 |
| Preoperative Decompression Side - Postoperative Decompression Side | 10.51 ± 59.68 | 0.088 |
| Preoperative Contralateral Side - Postoperative Contralateral Side | 5.77 ± 30.84 | 0.07 |
| Postoperative Contralateral Side - Postoperative Decompression Side | 1.85 ± 80.48 | 0.822 |
Table 4: Effective paraspinal muscle comparison. All statistical analyses were performed using paired-sample t-test.
Discussion
Over the past decade, MIS-TLIF, PE-TLIF, and BE-TLIF have gradually become alternatives to traditional open TLIF surgery, offering advantages in terms of trauma, blood loss, and postoperative recovery23. Some scholars even believe that endoscopic-assisted lumbar fusion surgery will eventually replace open surgery. However, our study shows that open TLIF surgery has also made significant progress in terms of minimally invasive techniques. While retaining the simplicity and wide applicability of traditional open procedures, it has achieved clinical outcomes and minimally invasive effects similar to endoscopic minimally invasive techniques.
Preoperatively, we mark the pedicle projection, puncture needle entry points, target segments intervertebral space, and surgical incision. However, we recommend verifying the segment through fluoroscopy again before decompression. This is because even a slight change in angle during dissection can lead to segmental errors, as we have encountered two such cases during our surgeries. After dissection, an ultrasonic bone scalpel is routinely used to perform osteotomy and expose the spinal canal. When dealing with the spinal canal, it is advisable to partially retain the ligamentum flavum to reduce scar adhesion, removing only the excessively thick portions that compress the nerves. If contralateral central canal decompression is needed, the surgical bed angle can be adjusted to remove part of the spinous process root bone, achieving decompression on the opposite side. Bilateral lateral recess decompression may require bilateral dissection. During decompression, for safety reasons, it is not necessary to fully expose the exiting root; a hook can be used to probe around the nerve root, and if there is sufficient space, extensive manipulation is not required. After completing the decompression and fusion, the central small incision can be closed, and percutaneous pedicle screws can be placed, which can shorten the retraction time on the paraspinal muscles. In terms of cage selection, MO-TLIF can use the same size fusion cages as traditional open TLIF without the need for smaller or expandable cages, which helps restore disc height and reduces the risk of cage subsidence24. A meta-analysis suggested that the straight cage occupies a greater area of the endplate than the banana-shaped cage, thus creating a better distribution of the pressure, which may contribute to a lower subsidence rate25. This study found only one case of cage subsidence during follow-up. MO-TLIF can also achieve over-the-top decompression to the contralateral side, making it suitable for unilateral laminotomy for bilateral decompression (ULBD). This effectively increases the spinal canal area, although further statistical analysis is needed to measure improvements in intervertebral height, lumbar angles, and spinal canal area.
Due to the small size of the central incision, it is not suitable for revision surgeries that require replacement of internal fixation. However, this approach remains applicable in most other cases. For instance, in surgeries involving four segments, two small central incisions can be used, with each incision handling the decompression of two segments.
The MO-TLIF technique can be performed under direct visualization or with the assistance of visual tools such as loupes or microscopes. In this study, the patients undergoing multi-level MO-TLIF showed good outcomes, with an average surgery time of 108.3 min and an average blood loss of 130.2 mL. The limited subperiosteal dissection and minimal muscle retraction may protect the paraspinal muscles, resulting in similar clinical outcomes and muscle impact as endoscopic-assisted lumbar fusion surgery.
In the foreseeable future, endoscopic or tubular techniques may not completely replace open surgical approaches. MO-TLIF can minimally invasive open surgery while retaining the unique advantages of open surgery, such as the convenience of multi-level operations, shorter operative time, a smooth learning curve, no need for specialized instruments, and water resource conservation. Research by Zhang et al. indicates that the operative time for PE-TLIF is 202 ± 31.4 min with blood loss of 73 ± 26.4 mL, which is significantly less than the intraoperative blood loss for MIS-TLIF (192 ± 18.9 min, 129 ± 31.7 mL), though the surgery time is longer26. In contrast, a study by Xue et al. found that the operative time for PE-TLIF (140.3 ± 35.6 min) is shorter than MIS-TLIF (170.6 ± 54.8 min), with intraoperative blood loss for PE-TLIF (65.6 ± 15.3 mL) being less than MIS-TLIF (140.5 ± 21.5 mL)27. The differences in surgery time may be related to the surgeon's proficiency. Meta-analyses show that the average operative time for PE-TLIF is 155 min with an average intraoperative blood loss of 101.1 mL, while MIS-TLIF has an average operative time of 181.1 min and intraoperative blood loss of 174 mL28,29.
The intraoperative blood loss for single-level MO-TLIF (64.5 ± 30.2 mL) is similar to that of PE-TLIF and better than MIS-TLIF, with a significantly shorter operation time (102.3 ± 17.2 min). MO-TLIF is advantageous for multi-level operations, with a 3 cm single-level incision extended by 1 cm proximally or distally for each additional level. This approach allows for decompression operations with minimal extension of the incision and only slight increases in blood loss and operation time.
In conclusion, while minimally invasive endoscopic techniques have clear advantages, the ongoing evolution of minimally invasive open procedures like MO-TLIF continues to offer unique benefits, maintaining their relevance and effectiveness in spinal surgery.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
This study was supported by a fund from the National Natural Science Foundation of China in 2021 (project number: 82474251) and a fund from the science and technology project of Suzhou Health Commission in 2024 (project number: LCZX202307).
Materials
| Name | Company | Catalog Number | Comments |
| Absorbable sutures | Suzhou Jiahe | VT401L | |
| Blade | KYUAN | T00100 | |
| C-arm fluoroscopy | Siemens | Siremobile Compact L | |
| High frequency electrotome | Zhejiang Huatong | 20162010692 | |
| Iodophor | Likang High-tech | 31005102 | |
| Lumbar fusion cage | Shandong Weigao | GJXT310417 | |
| Premier posterior spine minimally invasive nail rod system | Shandong Weigao | GJXT310417 | |
| Suture | MERSILK | SA86G | |
| Ultrasonic bone knife | SMTP Technology | XD860A |
References
- Souslian, F. G., Patel, P. D. Review and analysis of modern lumbar spinal fusion techniques. Brit J Neurosurg. 38, 61-67 (2024).
- Mobbs, R. J., Phan, K., Malham, G., Seex, K., Rao, P. J. Lumbar interbody fusion: Techniques, indications and comparison of interbody fusion options including plif, tlif, mi-tlif, olif/atp, llif and alif. J Spine Surg. 1 (1), 2-18 (2015).
- Foley, K. T., Gupta, S. K., Justis, J. R., Sherman, M. C. Percutaneous pedicle screw fixation of the lumbar spine. Neurosurg Focus. 10 (4), E10(2001).
- Zhao, J., Zhang, F., Chen, X., Yao, Y. Posterior interbody fusion using a diagonal cage with unilateral transpedicular screw fixation for lumbar stenosis. J Clin Neurosci. 18 (3), 324-328 (2011).
- Foley, K. T., Lefkowitz, M. A. Advances in minimally invasive spine surgery. Clin Neurosurg. 49, 499-517 (2002).
- Kambin, P. Arthroscopic microdiscectomy. Arthroscopy. 8 (3), 287-295 (1992).
- Osman, S. G. Endoscopic transforaminal decompression, interbody fusion, and percutaneous pedicle screw implantation of the lumbar spine: A case series report. Int J Spine Surg. 6, 157-166 (2012).
- Kim, J. E., Choi, D. J. Biportal endoscopic transforaminal lumbar interbody fusion with arthroscopy. Clin Orthop Surg. 10 (2), 248-252 (2018).
- Derman, P. B., Albert, T. J. Interbody fusion techniques in the surgical management of degenerative lumbar spondylolisthesis. Curr Rev Musculoskelet Med. 10 (4), 530-538 (2017).
- Meng, F. J., et al. Comparison research of mMO-TLIF via midline approach versus MIS-TLIF via Wiltse approach for thoracolumbar surgery. Orthop J China. 28, 118-122 (2020).
- Li, S. W., et al. Comparison of mMO-TLIF via midline incision versus MIS-TLIF via Wiltse approach in lumbar degenerative disease. Indian J Orthop. 58, 1278-1287 (2024).
- Anand, N., Hamilton, J. F., Perri, B., Miraliakbar, H., Goldstein, T. Cantilever tlif with structural allograft and rhbmp2 for correction and maintenance of segmental sagittal lordosis: Long-term clinical, radiographic, and functional outcome. Spine. 31 (20), E748-E753 (2006).
- Shafaq, N., et al. Asymmetric degeneration of paravertebral muscles in patients with degenerative lumbar scoliosis. Spine. 37 (16), 1398-1406 (2012).
- Laasonen, E. M. Atrophy of sacrospinal muscle groups in patients with chronic, diffusely radiating lumbar back pain. Neuroradiology. 26 (1), 9-13 (1984).
- Hira, K., et al. Relationship of sagittal spinal alignment with low back pain and physical performance in the general population. Sci Rep. 11 (1), 20604(2021).
- Hiyama, A., et al. The correlation analysis between sagittal alignment and cross-sectional area of paraspinal muscle in patients with lumbar spinal stenosis and degenerative spondylolisthesis. BMC Musculoskelet Disord. 20 (1), 352(2019).
- Singh, K., et al. A perioperative cost analysis comparing single-level minimally invasive and open transforaminal lumbar interbody fusion. Spine J. 14 (8), 1694-1701 (2014).
- Phan, K., Rao, P. J., Kam, A. C., Mobbs, R. J. Minimally invasive versus open transforaminal lumbar interbody fusion for treatment of degenerative lumbar disease: systematic review and meta-analysis. Eur Spine J. 24 (5), 1017-1030 (2015).
- Nadler, S. B., Hidalgo, J. H., Bloch, T. Prediction of blood volume in normal human adults. Surgery. 51 (2), 224-232 (1962).
- Gross, J. B. Estimating allowable blood loss: corrected for dilution. Anesthesiology. 58 (3), 277-280 (1983).
- Bridwell, K. H., Lenke, L. G., Mcenery, K. W., Baldus, C., Blanke, K. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Spine. 20 (12), 1410-1418 (1995).
- Jacob, K. C., et al. The effect of the severity of preoperative disability on patient-reported outcomes and patient satisfaction following minimally invasive transforaminal lumbar interbody fusion. World Neurosurg. 159, E334-E346 (2022).
- Ba, Z., et al. Percutaneous endoscopical transforaminal approach versus plf to treat the single-level adjacent segment disease after plf/plif: 1-2 years follow-up. Int J Surg. 42, 22-26 (2017).
- Zhang, H., et al. Percutaneous endoscopic transforaminal lumbar interbody fusion: Technique note and comparison of early outcomes with minimally invasive transforaminal lumbar interbody fusion for lumbar spondylolisthesis. Int J Gen Med. 14, 549-558 (2021).
- Amer, S., Gaby, K., Jean, T., Khalil, K., Mohammad, D. Transforaminal lumbar interbody fusion using banana-shaped and cages: meta-analysis of and outcomes. Eur Spine J. 32, 3158-3166 (2023).
- Xue, Y. D., Diao, W. B., Ma, C., Li, J. Lumbar degenerative disease treated by percutaneous endoscopic transforaminal lumbar interbody fusion or minimally invasive surgery-transforaminal lumbar interbody fusion: A case-matched comparative study. J Orthop Surg Res. 16 (1), 696(2021).
- Zhu, L., et al. Comparison of clinical outcomes and complications between percutaneous endoscopic and minimally invasive transforaminal lumbar interbody fusion for degenerative lumbar disease: A systematic review and meta-analysis. Pain Physician. 24 (6), 441-452 (2021).
- Song, Y. F., et al. Percutaneous endoscopic versus minimally invasive transforaminal lumbar interbody fusion for lumbar degenerative diseases: A meta-analysis. Wideochir Inne Tech Maloinwazyjne. 17 (4), 591-600 (2022).
- Aoki, Y., et al. Influence of pelvic incidence-lumbar lordosis mismatch on surgical outcomes of short-segment transforaminal lumbar interbody fusion. BMC Musculoskelet Disord. 16, 213(2015).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved