Method Article
A High-Fidelity Porcine Model of Orthotopic Heart Transplantation Following Donation after Circulatory Death
* These authors contributed equally
In This Article
Summary
The protocol here describes a high-fidelity porcine model of heart transplantation following donation after circulatory death utilizing ex vivo perfusion of the allograft.
Abstract
The number of advanced heart failure patients who can receive a heart transplant is limited by a shortage of suitable organ donors. In efforts to expand the donor pool, alternative donation and procurement methods have been developed, including heart transplantation following donation after circulatory death (DCD HT). While short-term survival following DCD HT is non-inferior to heart transplantation with brain-dead donors, there may be an increased rate of primary graft dysfunction (PGD) associated with DCD HT allografts. The underlying etiology of PGD is multifactorial and incompletely understood. For DCD HT allografts, the period of warm ischemic injury during DCD procurement is a potential risk factor for PGD to which brain death allografts are not exposed. The functional warm ischemic time thus may be an important driver of PGD in DCD HT. However, the mechanisms underlying PGD in this clinical scenario are poorly understood at the molecular level. The work presented herein aims to describe the development and validation of a high-fidelity non-survival porcine model of DCD orthotopic heart transplantation. We hypothesize that the use of this translational large animal model is critical to elucidate molecular mechanisms contributing to PGD, as well as to investigate interventions designed to optimize allograft preservation and early performance. This model replicates the perioperative and surgical approach used in DCD HT clinically, with modifications to account for porcine anatomy and physiology. The development of this large animal surgical model will not only provide mechanistic insights into the development of PGD but also can be modified to enhance translational research efforts aimed at improving organ recovery following DCD HT.
Introduction
For patients with end-stage heart failure refractory to medical management, heart transplantation remains the therapy associated with the best long-term survival and quality of life. Historically, heart transplantation required the use of a heart allograft procured from a brain-dead donor (DBD HT) and transported while preserved with hypothermic static storage. However, the number of patients who require a heart transplant exceeds the number of available donors. While more than 5,000 heart transplants are performed annually worldwide, it is estimated that 50,000 candidates await a heart transplant1. In addition, the utilization of organs from identified donors remains as low as 30%2. In order to improve donor organ utilization, alternative donation and procurement methods have been developed in recent years, including heart transplantation following donation after circulatory death (DCD HT)3,4,5,6.
DCD HT donors do not meet formal brain-death criteria but have a non-recoverable neurologic injury for which ongoing medical care is deemed futile. During a DCD HT procurement, life-sustaining measures are withdrawn, and the patient is monitored for progression to apnea and circulatory arrest. Death in these situations is declared by a physician not participating in the transplant or organ procurement process. Once death has been declared, there is an additional standoff period (usually 5 min) where the potential donor is observed to ensure there is no recovery or signs of life, after which declaration of death is reconfirmed prior to proceeding with organ procurement7,8. DCD allografts are, therefore, exposed to a variable period of warm ischemia (at least 10 min) to which DBD donor allografts are not exposed. This period of warm ischemia previously deterred the use of heart transplantation with DCD donors. However, within the last five years, two procurement reperfusion methods were developed for allograft recovery following the warm ischemic time associated with DCD. First, direct procurement and perfusion (DPP) involves donor cardiectomy upon the confirmation of circulatory death, followed by allograft resuscitation and preservation by ex vivo perfusion. Alternatively, normothermic regional perfusion (NRP) uses extracorporeal circulation with the exclusion of the cerebral circulation to reperfuse and reanimate the donor heart in situ prior to cardiectomy9,10,11,12.
Thus far, short-term survival associated with DCD HT has been similar to that observed with DBD HT. However, early studies also suggest there is a higher risk of severe primary graft dysfunction (PGD) with DCD HT compared to DBD HT3. PGD is a term used to describe the clinical circumstance where, following heart transplantation, early allograft function is insufficient to meet the recipient's circulatory needs. A system for grading the severity of PGD was described by the International Society of Heart and Lung Transplantation. In severe PGD, mechanical circulatory support is required to support adequate circulation in the post-transplant period13. This condition is the main driver of early postoperative mortality after heart transplantation. The etiology of PGD for both DBD HT and DCD HT is poorly understood but is likely multifactorial, with donor, preservation, and recipient variables all contributing. For DCD allografts, the warm ischemic injury during DCD procurement, as well as deleterious donor-specific responses, including hemodynamic instability, metabolic derangements, and surges in catecholamines, cytokines, lactate, and potassium, are proposed factors that may contribute to an increased risk of PGD compared to DBD allografts. 14,15,16. However, the mechanisms underlying PGD in this clinical scenario are poorly understood at the molecular level. In addition, perhaps owing to these concerns, DCD hearts are 3.37 times more likely to be declined than DBD organs17. As a result, there is still an unmet need to enhance organ utilization and improve transplant outcomes by optimizing the organ preservation process.
In this study, we describe a porcine DCD HT using DPP that mirrors current clinical DPP DCD HT with high fidelity. This model includes elements throughout the DCD transplant process: 1) DCD procurement, 2) ex vivo perfusion preservation with an Organ Care System (OCS), and 3) recipient implantation. This comprehensive model provides an opportunity to better characterize mechanisms underlying PGD in DCD HT. This then allows for the development of targeted and rational therapeutic strategies aimed at improving allograft preservation and performance. Furthermore, this model provides a platform for the preclinical evaluation of such novel therapeutics, which will be important to help advance the field of heart transplant in a safe and expeditious manner.
Protocol
All husbandry and procedures were approved by Duke University Medical Center's Institutional Animal Care and Use Committee in line with their regulations and guidelines.
1. Preoperative donor preparation
- Select two blood-type matched Yorkshire pigs weighing 70-80 kg (blood typing performed by Looper Farms in Granite Falls, NC, USA). Fast both pigs for a minimum of 6-8 h prior to surgery.
- Prepare the heart perfusion module according to the manufacturer's guidelines and the necessary point-of-care testing (Table of Materials).
- Prepare the maintenance solution for the heart perfusion module. This solution contains nutrients for the metabolically active heart, as well as adenosine, to aid in maintaining the target blood pressure.
- Add the following: 500 mL of clear priming solution, 100 mL of 25% albumin, 100 mg of ciprofloxacin (200 mg/100 mL), two 5 mL vials of multivitamin injection, 20mEq sodium bicarbonate, 1 g of cefazolin sodium, and 250 mg of methylprednisolone. Spike the priming solution and add the contents to the heart perfusion module.
- Once prepared, inject 50 IU human recombinant insulin into the bag.
- Prepare an epinephrine solution by injecting 0.25 mg of epinephrine and 30 IU human recombinant insulin into a 500 mL bag of 5% dextrose in water. This solution is meant to replace the catecholamines rather than have an inotropic effect. Infuse the solution at 10 mL/h.
- Prepare the T4 solution by injecting 200 µg of reconstituted levothyroxine into a 100 mL 0.9% normal saline bag. Infuse at a rate of 3 mL/h.
- Prepare the maintenance solution for the heart perfusion module. This solution contains nutrients for the metabolically active heart, as well as adenosine, to aid in maintaining the target blood pressure.
- Sedate the designated donor pig with 4.4 mg/kg of telazol and 1.0 mg/kg of propofol followed by 2%-5% inhaled isoflurane delivered by face mask.
- While the pig is in the supine position, intubate with an 8-10 mm endotracheal tube for maintenance of airway during surgery. Secure the endotracheal tube in place to the snout. Apply veterinary ointment to the eyes to maintain ocular lubrication during the surgery.
- Insert an intravenous (IV) catheter into an ear vein to administer IV fluids (maintenance fluids: Lactated Ringer's solution 10 mL/kg/h).
- Begin continuous fentanyl infusion for analgesia (25-100 µg/h) and administer 0.2 mg/kg of vecuronium to maintain paralysis.
- Place electrocardiogram leads and pulse oximeter for continuous monitoring of electrical activity and oxygenation.
- Begin mechanical ventilation at a tidal volume of 10 mL/kg/min, a rate of 10-15 breaths per minute, and with maintenance of isoflurane (1%-5%) for the remainder of the procedure. Titrate anesthetics and vasoactive medications such that reflexes are absent, heart rate remains 61-99 bpm, and systolic blood pressure remains 90-130 mmHg.
- Position the pig on the operating table with the upper extremities positioned cephalad and secured outside the operative field. Secure the lower extremities outside of the operative field.
- Define the area to be included in the operative field by the jowl superiorly, anterior axillary line bilaterally, and two finger breadths below the xiphoid process inferiorly. Prep and drape the surgical site aseptically, following standard practices.
- Perform a right-sided carotid cut-down.
- Using a #10 blade, make an oblique right lateral neck incision, two finger breadths lateral to the trachea, along the medial border of the sternocleidomastoid muscle.
- Carry this incision down through the platysma with electrocautery, ensuring hemostasis. Once through the platysma, use a self-retaining retractor to aid exposure.
- Carefully dissect and isolate the carotid artery and internal jugular vein; place a vessel loop around each vessel.
- Apply 10 mL of 2% lidocaine topically to bathe the vessels.
NOTE: Lidocaine is used for its vasodilatory effects, as the porcine vessels easily spasm with manipulation and can become difficult to cannulate. - Using the Seldinger technique, directly cannulate the carotid artery with a 5 Fr micropuncture, followed by a 6 Fr introducer sheath.
- Connect the introducer sheath to an arterial line and transducer for continuous monitoring of systemic arterial pressure during the procedure.
- Draw blood from the carotid artery arterial line for preoperative laboratory bloodwork (complete blood count, comprehensive metabolic panel, troponin, lactate, and whole blood for peripheral blood mononuclear cell isolation).
- Cannulate the internal jugular vein using the same technique as in 1.11.5. Leave the 6 Fr sheath in place, as this will be used to introduce a pressure-volume loop catheter for downstream functional assessments.
2. Donation after circulatory death and donor cardiectomy
- Perform a sternotomy.
- Using a #10 blade, make a longitudinal incision from the sternal notch to the xiphoid process. Using electrocautery, carry the dissection down through the subcutaneous tissue and pectoralis muscle fascia.
- Score the midline of the sternum with electrocautery. Dissect the ligamentous attachments at the manubrium and around the xiphoid process.
- Use an oscillating sternal saw to carefully divide the anterior table of the sternum along the midline. Use the oscillating saw to completely divide the posterior table of the sternum at the level of the manubrium. Take care to avoid injury to the innominate vein at this step. Complete the sternal division of the posterior table below the manubrium using a pair of heavy scissors.
NOTE: Manual blunt dissection of the plane between the underside of the sternum and the pericardium can help avoid injury to the heart during this step. - Use electrocautery to obtain hemostasis of the sternal edges. Place a sternal retractor.
- Remove the thymic and pericardial fat pad tissue with electrocautery to optimize exposure of the pericardium.
- Carefully open the pericardium with electrocautery. Carry the incision longitudinally from the apex of the heart to the level of the aorta.
- Create a pericardial well with 2-0 silk stay stitches.
- Perform baseline donor allograft analyses.
- While the donor heart is exposed and prior to any direct cardiac manipulation, perform baseline analyses. These include direct epicardial echocardiography, pressure-volume loop assessment, and myocardial core needle biopsies.
- Perform echocardiography in a sterile fashion to obtain standard long and short-axis views directly from the epicardial surface.
- In order to conduct pressure-volume loop recordings, introduce a solid-state pressure-volume catheter (5Fr, 122 cm) sequentially into the carotid artery and internal jugular vein into the left and right ventricles, respectively.
- Measure the length of the catheter to be introduced externally (from the cannulation site to the apex of the heart) prior to insertion into the sheath.
- Introduce the catheter into the arterial sheath, guiding it into the left ventricle using epicardial ultrasound. Ensure the catheter is not directly touching a ventricular wall, as this may interfere with measurement accuracy.
- Next, introduce the catheter into the venous sheath, guiding it into the right ventricle, using direct palpation or epicardial ultrasound guidance.
- Perform core needle myocardial biopsies to collect tissue for later analyses. Perform this in the apex of the left ventricle. If there is significant bleeding from the biopsy site, place a repair stitch.
- Prepare the donor heart.
- Dissect the aortopulmonary window to allow space for cross-clamp placement.
- Circumferentially dissect the superior vena cava (SVC) and inferior vena cava (IVC) with electrocautery.
- To anticoagulate, administer 300 units/kg of heparin intravenously (IV) prior to aortic cannulation.
- Use a double-armed 4-0 polypropylene suture to place an aortic purse-string in the aortic root for aortic cannulation. Cannulate with 7 Fr (14G) aortic root vent cannula.
- De-air and secure in place with a Rumel tourniquet. Flush the cardioplegia tubing to de-air and secure the line to the aortic root cannula.
- Next, to prepare for future venous cannulation, place a purse-string stitch in the right atrial appendage and secure it with a Rumel tourniquet.
- Perform controlled circulatory death.
- Provide a 250 µg of fentanyl IV bolus followed by continuous rate infusion (CRI) at 100 µg/h for analgesia. To paralyze, administer 0.2 mg/kg vecuronium intravenously.
- Cease mechanical ventilation to begin the controlled circulatory death process.
- Continually monitor systolic and mean arterial blood pressure, arterial line pulsatility, electrical cardiac activity, and oxygenation status.
NOTE: Agonal phase begins when the systolic blood pressure falls below 50 mmHg. At this point, the heart may distend. Death is defined by mechanical asystole (pulseless electrical activity) and no signs of life. This study does not define death by electrical asystole in this model, as slow pulseless electrical activity persists in swine for a longer time period than observed in humans, which may result in increased allograft ischemic insult than intended for the model. - Wait for 10 min after the time of death/asystole prior to proceeding with the following steps of the protocol. This interval simulates the standoff period plus the time needed for mediastinal entry and preparation in the clinical DCD HT.
- Perform ex vivo perfusion device priming.
- With an #11 blade, make a stab incision into the right atrial appendage and cannulate with the 34Fr venous cannula provided by the manufacturer. Secure the cannula with a Rumel tourniquet and connect the cannula to the ex vivo perfusion device's collection bag.
- Collect approximately 1200-1500 mL of donor blood into the collection bag provided by the manufacturer. Prior to exsanguination, 30,000 U of heparin and 2 mg of tirofiban HCl will have been added to the collection bag.
- Filter the donor blood and deliver it into the heart perfusion module.
- Perform donor cardiectomy.
- Following the collection of the donor blood, apply the aortic cross-clamp.
- Note that the swine aortic root and ascending aorta are considerably shorter than in humans. Take care to apply the cross-clamp as proximally as possible in order to avoid clamping the arch or incompletely occluding the aorta.
- Administer 1 L of Del Nido cardioplegia into the aortic root, targeting a root pressure of 60-100 mmHg.
- Transect the inferior vena cava at the pericardial reflection and the left atrial appendage to vent the right and left ventricles during cardioplegia delivery.
- Place cold sterile slush on the donor heart.
- Divide the IVC, SVC, aorta just distal to the innominate artery, and pulmonary artery (PA) at the bifurcation. Transect the donor left atrium (LA), leaving a sufficient cuff of tissue on the allograft.
- Remove the heart from the field and place it in a basin filled with sterile, cold slush for back-table preparation.
- Place four equidistant pledgeted horizontal mattress sutures (4-0 polypropylene) inside the distal end of the aorta.
- Following the collection of the donor blood, apply the aortic cross-clamp.
- Ex vivo perfusion
- Place the ex vivo perfusion aortic adapter into the distal aorta and secure tightly with a 0-0 silk suture or umbilical tape placed just below the pledgeted sutures.
- Leave the PA, SVC, IVC, and left atrium open while on the ex vivo perfusion device.
NOTE: With this configuration, no left ventricular distension was encountered. - Transport the allograft to the ex vivo perfusion device and connect the aortic adapter. Ensure that the posterior surface of the heart is facing anteriorly. Ensure that the anterior surface of the heart is in contact with the device and two defibrillation pads. Maintain the allograft at 34 °C. Defibrillate as needed to restore any ventricular arrhythmia to an organized rhythm.
- At this point, a therapeutic could be introduced into the ex-vivo perfusion circuit through the cardioplegia port. Alternatively, the perfusate or physical circuit can be modified to evaluate experimental conditions aimed at enhancing recovery of a DCD HT allograft. For example, we have previously shown that the perfusate needs to be washed of pre-formed neutralizing antibodies via a cell-saver to enhance transduction with viral vectors18,19,20.
- Use point-of-care labs to guide the administration of sodium bicarbonate, calcium gluconate, and dextrose as needed.
- Obtain perfusate samples, bloodwork, and core needle biopsy samples during this ex vivo perfusion stage.
NOTE: In this model, the allograft remains on the ex vivo perfusion device for approximately 2-3 h prior to implantation into the recipient (Table 1). The length of time the allograft was perfused ex vivo was determined by the length of time it took to prepare the recipient animal for implantation. However, the ex vivo perfusion time is a variable that can be adjusted based on study objectives.
3. Recipient cardiectomy, implantation, and reperfusion
- Prepare the recipient pig as described in steps 1.3-1.11.8.
- In addition, place a 7 Fr 20 cm triple lumen central line catheter into the left external or internal jugular vein using ultrasound guidance and the Seldinger technique. Place a 5 Fr femoral arterial line using ultrasound guidance.
- After draping the sterile field, prepare the cardiopulmonary bypass (CPB) circuit and bring the lines onto the field.
- Recirculate through the CPB arterial and venous lines to de-air. Clamp and divide the lines. Prepare the venous line for bicaval cannulation.
- Administer a preoperative dose of 1 g of methylprednisolone to address any inflammatory responses due to rejection or ischemia, as is done in clinical heart transplantation at the institution where this study is performed.
- Perform a right carotid cut down and midline sternotomy as previously described in steps 1.11 and 2.1.
- Perform recipient cardiectomy.
- Dissect the aortopulmonary window as before. Dissect the SVC and IVC and place circumferential umbilical tape around the vessels.
- Prior to cannulation, administer 400 units/kg of heparin. Titrate to a goal of activated clotting time (ACT) > 480 s.
- Place a standard aortic cannulation purse-string stitch in the distal ascending aorta (using double-armed pledgeted 2-0 coated braided polyester suture). Secure with a Rumel tourniquet and hemostat.
- Ensure systolic blood pressure is less than 120 mmHg. Use a #11 blade to make a full-thickness stab incision within the purse-string. Cannulate the aorta with a 15 Fr cannula through this incision. Secure the cannula in place with a Rumel tourniquet.
- Clamp the aortic cannula. After de-airing the arterial bypass line, connect the aortic cannula to the CPB circuit. Ensure no air bubbles are present in the line.
- Make another aortic purse-string and insert the aortic root vent cannula.
- Perform venous cannulation using a bicaval technique with 18-20 Fr right angle metal tip cannulas in both the SVC and IVC, secured with Rummel tourniquets. Then, connect these cannulas to the venous limb of the CPB circuit.
- Initiate CPB to a target flow of 2.4 L/min/m2 and cool to 32 °C. Titrate anesthetic and vasopressors to maintain a mean arterial pressure > 60 mmHg.
NOTE: These procedures are performed with board-certified cardiothoracic anesthesiologists and certified perfusionists who monitor and coordinate hemodynamic management throughout the course of the CPB run. - Apply the aortic cross-clamp proximal to the aortic cannula and proceed with an explanation of the recipient's native heart. Leave intact right and left atrial cuffs large enough to facilitate a bi-atrial implantation technique.
NOTE: The bi-atrial technique is used in this model as the porcine vasculature is very delicate, which makes the IVC and SVC vascular anastomoses technically challenging in a bicaval technique. - Transect the aorta and PA as close to the root as possible to maintain maximal vessel length. Place the newly explanted heart in ice-cold phosphate-buffered saline (PBS) to be transported and processed in the lab.
- Perform recipient allograft bi-atrial implantation.
- Cool the allograft on the ex vivo perfusion device as per manufacturer instructions. Administer 1 L of cold Del Nido cardioplegia for cardioplegic arrest once again prior to removal from the device.
- Disconnect the aortic adaptor and transport the heart in a basin of sterile, cold slush to the back table.
- Inspect the heart for any patent primum fossa ovalis (PFO) or sites of inadvertent injury.
NOTE: Importantly, in swine, there is usually a systemic vein that drains along the left atrial border before draining into the right atrium or IVC. This vein may be opened in the procurement process, and care should be taken to ensure that it is ligated prior to proceeding with implantation. - Trim the aorta back to healthy tissue below the adaptor securement site.
- Using Metzenbaum scissors, trim the left atrial opening to match the size of the recipient LA cuff. Do the same to the right atrium.
- Begin the left atrial anastomosis in a running fashion with double-armed 4-0 Prolene suture. Continue with the right PA, aortic, and right atrial anastomoses, also all completed with continuous 4-0 prolene suture.
- Begin rewarming to normothermia (37 °C) prior to removal of the aortic cross-clamp.
- Perform allograft reperfusion and wean from CPB.
- After completing all anastomoses, release the aortic cross-clamp to reperfuse the allograft.
- Ensure hemostasis of all anastomoses.
- Await the return of native rhythm and contractility. Place epicardial pacing wires and pace at 100 bpm if the allograft heart rate is <100 bpm.
- After 60 min of reperfusion, attempt to wean from CPB. The recipients are supported with 0.05 µg/kg/min of epinephrine as well as vasopressor infusions as needed to maintain a mean arterial pressure (MAP) >65 mmHg.
- Perform analyses such as echocardiography, bloodwork, myocardial biopsies, and pressure-volume loop assessment at both early and late reperfusion time points.
4. Termination of the experiment and euthanasia
NOTE: In this study, the transplant recipient animal was supported for 1 h after separation from CPB.
- Upon completion of the experiment, euthanasia is performed via exsanguination under anesthesia.
- Explant the transplanted allograft by transecting the suture lines along all anastomoses. Take care not to include any native recipient tissue with the allograft specimen.
- Place the allograft immediately into ice-cold PBS and transport it to the laboratory for processing and storage.
Results
A total of 6 porcine DCD transplants were performed for a pilot analysis using the protocol described here. Over the course of these six pilot experiments, components of the protocol were refined to better suit the needs of the model, enhance reproducibility, and account for logistical constraints. The final resulting protocol, as written here, is summarized in Figure 1.
Unlike in the human-controlled circulatory death process, porcine hearts subjected to ischemia sustained electrical activity for much longer than expected, even after contractility ceased. Upon recognition of this difference in the first experiment, electrical asystole was deemed to be unreliable as a measure for declaration of death. In order to avoid warm-ischemic damage to the allograft out of proportion to that seen in the clinical context, mechanical asystole (pulseless electrical activity) was utilized to determine the time of death for the remaining transplant procedures.
Additionally, the standoff period was initially 15 min prior to proceeding with donor cardiectomy. This was utilized for the first three experiments of the pilot set. Part of the rationale for this 15 min warm ischemia period was that we were aiming to have severe enough injury to have a degree of post-transplantation graft dysfunction that would be suitable for study. We found that graft dysfunction in these early experiments was so severe that we had difficulty sustaining the recipient animal for an hour following separation from CPB, such that the recipient animal required high doses of inotropic and pressor support to meet the 1-h timepoint. Given these difficulties, we reduced the period of warm-ischemic injury to 10 min, which more closely approximates the clinical DCD HT with ex vivo perfusion. Figure 2 depicts representative images of the allograft at this stage as well as during ex vivo perfusion and after implantation. Lastly, adjustments were made to the titration of vasopressor and inotrope infusions in the post-CPB wean phase to support the newly transplanted heart. The need for an experienced team with specific expertise in cardiovascular anesthesiology to enhance success in this post-CPB phase cannot be emphasized enough.
Operative times were tracked across 4 of the 6 experiments and are summarized in Table 1. In the DCD procurement phase of the protocol, the elapsed time from cessation of life-sustaining measures in the donor pig to the declaration of death was 14.25 (+/- 2.6) min. Operative cardiopulmonary bypass time and cross-clamp time remained consistent throughout the pilot experiments at about 3 h and 1.5 h, respectively. Experiments were ended with euthanasia approximately 1 h after separation from CPB. There was variability in the degree of stability of the animals at the time of euthanasia. Some animals demonstrated relatively good stability and only mild graft dysfunction, while others demonstrated significant hemodynamic instability and severe graft dysfunction. Representative still echocardiography displays are demonstrated in Figure 3. This and other functional assessments, such as PV loops, can be utilized to determine differences in allograft function from baseline as well as before and after the introduction of therapeutic interventions.
A summary of samples collected, processed, and stored through the experiment is displayed in Figure 4. Explanted hearts were immediately placed in ice-cold PBS and stored in the lab for tissue and molecular analysis. The recipient's native heart was utilized as a control, while the transplanted allograft was stored as the experimental tissue. The hearts were cut into 4 cross-sections from apex to base. From each of these, representative tissue samples of each chamber (left ventricle, right ventricle, septum, and both atria) were flash-frozen in liquid nitrogen and stored at -80 °C for future analysis. Similarly, representative tissue samples from each of these levels and chambers were incubated in RNAlater and flash-frozen. The remaining sample of tissue was preserved in formalin for histopathologic analysis. Blood samples from any time point were obtained in duplicate and stored in either EDTA or CPT tubes. Blood stored in EDTA tubes was spun down to isolate plasma, which was flash-frozen. Blood in the CPT tubes was processed for PBMCs using a modified protocol provided by the CPT tube vendor.
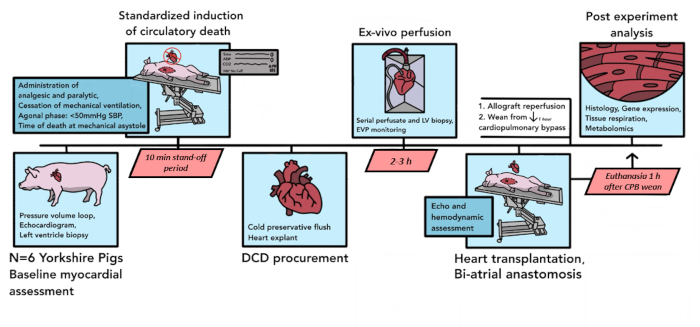
Figure 1: Porcine DCD HT protocol schematic. The timeline of events that occur in the porcine DCD HT procedure is depicted here. In this pilot study, 6 DCD HTs were conducted. Baseline myocardial assessments are performed on the donor pig heart allograft, after which controlled circulatory death commences. After the declaration of death, there is a 10-min standoff period. The allograft is then explanted and transported to the ex vivo perfusion device, where it is reperfused for 2-3 h. After the preparation of the recipient animal, the donor allograft is implanted with a biatrial technique. Following 1 h of reperfusion on CPB, the recipient is weaned from bypass support. Euthanasia occurs 1 h after separation from CPB. The implanted allograft is then processed for tissue analysis. DCD HT = heart transplantation following donation after circulatory death; CPB = cardiopulmonary bypass. Please click here to view a larger version of this figure.
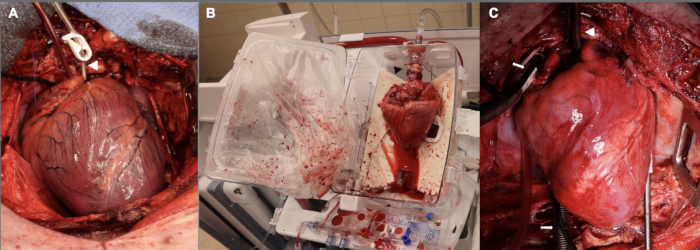
Figure 2: Porcine cardiac allograft at different stages along the DCD OHT process. (A) Following controlled circulatory death, the donor allograft is distended, ischemic, and edematous. The white arrowhead shows the aortic root cannula. (B) Once the allograft is explanted from the donor, it is placed on the EVP device for ex vivo perfusion. Note the orientation of the heart, with the posterior aspect facing outwards. The black arrowhead points to the aortic adaptor used to connect the allograft to the device. (C) After implantation in the recipient, the allograft is reperfused on cardiopulmonary bypass for one hour prior to weaning from CPB. The white arrowhead shows the aortic cannula; white arrows point to the bicaval venous cannulas. EVP = ex vivo perfusion; CPB = cardiopulmonary bypass. Please click here to view a larger version of this figure.
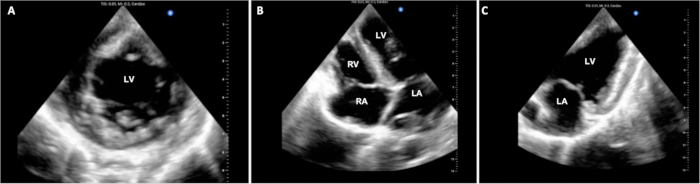
Figure 3: Cardiac epicardial echocardiography. Throughout the DCD OHT procedure, multiple epicardial echocardiographic images were acquired to evaluate ventricular function. (A) Standard short-axis, (B) 4-chamber, and (C) 2-chamber views are shown here. LV = left ventricle; LA = left atrium; RV = right ventricle; RA = right atrium. Please click here to view a larger version of this figure.
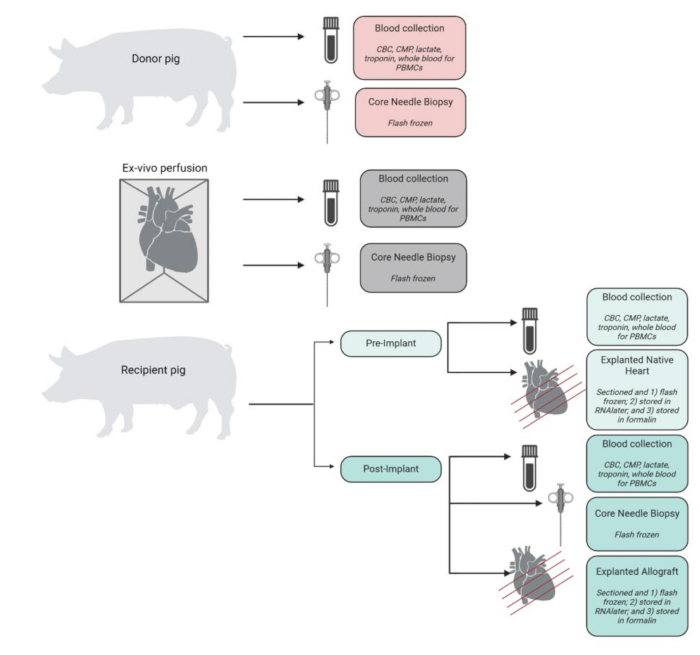
Figure 4: Specimen collection and processing workflow. Schematic of sample collection and processing at each step of the procedure. CBC = complete blood count; CMP = comprehensive metabolic panel; PBMCs = peripheral blood mononuclear cells. Figure created in BioRender. Please click here to view a larger version of this figure.
| Length of Time (min) | |
| (mean ± SD; n = 4) | |
| Time from cessation of ventilation to declaration of death | 14.25 (2.6) |
| Ex vivo perfusion duration | 147 (18) |
| CPB duration | 174 (4) |
| Cross-clamp time | 90 (12.5) |
| Time from CPB wean to death | 50 (37) |
Table 1. Procedural information. The average duration of each critical step in the DCD HT procedure. CPB = cardiopulmonary bypass.
Discussion
Despite the efficacy of heart transplantation for the treatment of end-stage heart failure, significant challenges persist in this field. To combat the limited availability of donor organs, advancements in heart allograft preservation methods (e.g., normothermic machine perfusion) have risen to the forefront in recent years. These advancements led to the adoption of the transplantation of cardiac allografts following donation after circulatory death (DCD HT). While utilization of DCD HT allografts expanded the donor pool and has short-term outcomes that are non-inferior to DBD HT allografts, there remains an approximately 5% risk of early peri-transplant mortality associated with both donor types3. The early mortality risk in heart transplantation is driven predominantly by PGD. While PGD is multifactorial in etiology with some contribution of defined donor, recipient, and preservation variables, the molecular mechanisms underlying PGD remain generally poorly understood. Furthermore, given the added warm ischemic injury incurred by the DCD HT process, it is not surprising that these hearts, compared to DBD allografts, may be at higher risk of PGD. Therefore, a better understanding of PGD is important to help reduce short-term mortality risk after orthotopic heart transplant, and this may be particularly true for DCD HT.
Here, we describe a high-fidelity porcine surgical model of DCD HT. The benefits of modeling DCD HT in vivo in a large animal model include not only the ability to further understand the pathophysiologic changes that occur in an allograft procured during DCD HT but also the ability to test targeted interventions aimed at ensuring optimal allograft quality. Evaluation of post-implantation left ventricular function, and pressure-volume loop analysis suggests that this porcine surgical model is able to recapitulate early cardiac allograft dysfunction following DCD HT. Thus, the technique detailed here creates a reproducible large animal model of PGD following DCD HT and is amenable to evaluating therapeutic strategies at many points along the DCD HT process. In fact, the use of ex vivo perfusion as a means for delivering novel therapeutics, such as viral vector-mediated gene therapy, is an active area of focus in our lab and others18,19,20. We previously demonstrated the ability to robustly and homogenously deliver a transgene to a cardiac allograft using a porcine non-DCD HT model; the same techniques can be applied to a DCD HT model19,20. Other potential therapeutic strategies include chemical modification of the perfusate, small molecule delivery aimed at decreasing oxidative stress, providing metabolic substrates to support metabolism during the ischemic period, etc16,21,22. Furthermore, the model presented herein can easily be adapted to evaluate different variables associated with DCD HT, including the different procurement techniques used clinically. For example, we describe a direct procurement and ex vivo perfusion strategy; however, the model can easily be adapted to incorporate alternate procurement perfusion methods, including normothermic regional perfusion.
The surgical technique employed in this model closely mirrors that used in the clinical setting; however, there are some key differences. First, the sternotomy and cardiac exposure occur prior to the cessation of cardiopulmonary support, agonal phase, determination of death, and ethically mandated standoff period (open-chest model)23. The sternotomy is done in this order so that baseline donor heart evaluation, including myocardial biopsies, can occur. Prior evaluation of the timing of sternotomy in a DCD HT porcine model demonstrated that the progression from withdrawal of life-sustaining measures to death (warm ischemic time) is more rapid with accompanying less pronounced hemodynamic changes in the open-chest model; these allografts may experience less-damaging conditions during procurement. However, there were no significant differences in biochemical (lactate, glucose, catecholamine, etc.) lab values or markers of cell death between the closed and open-chest groups24. Furthermore, due to the inclusion of the 10-min standoff period in the model, the time from withdrawal of ventilation to functional warm ischemia in this model more closely resembles the timing of the cited study's closed-chest group. Additionally, within this model, the time of functional warm ischemia is a variable that can be modified to fit experimental goals for a particular study. Another potential difference between the reported model here and the clinical setting is that pulseless electrical activity (PEA; mechanical asystole) is used to define death in this model. In the clinical context, a declaration of death is made by a physician who is not involved in the transplant process; either PEA, along with the absence of other signs of life or electrical asystole, would be acceptable for meeting the criteria for death declaration. The porcine hearts sustained electrical activity for a prolonged period following cessation of contractility. In order to avoid warm ischemic damage that is out of proportion to that seen in the clinical context, mechanical asystole (PEA) was used to define death. For investigators using a closed-chest model of DCD, pulseless electrical activity (lack of pulsatility on the arterial line) can still be used as the death-defining criteria. We did not find that peripheral oxygen saturation was a relevant marker to define the onset of the agonal period or to define death.
Lastly, what we have described herein is a time and resource-intensive model. From preparation of the donor pig to the death of the recipient animal takes approximately 10 h and requires a large collaborative effort. The procedural team includes experienced cardiac surgeons, cardiac anesthesiologists, perfusionists, and veterinary and laboratory staff who help with sample collection and processing. A large team such as this is integral during the complex procedure of DCD HT. However, with an experienced team, the creation of a high-fidelity porcine model of DCD HT is possible, as described within this manuscript.
Disclosures
CAM has received stock compensation for serving as a consultant for TransMedics Inc.
Acknowledgements
We thank the veterinary technical support from the Duke Laboratory Animal Resources, perfusion support from Centrifugal Solutions, and the Duke cardiovascular anesthesia teams for their invaluable support of these surgical experiments. We also sincerely acknowledge Paul Lezberg and TransMedics, Inc. for their support.
Materials
| Name | Company | Catalog Number | Comments |
| 0-0 silk suture with needle | DemeTECH | SK260026B0P | |
| 0-0 silk ties | DemeTECH | SK6X2600 | |
| 1/4" x 1/4" straight connector | Liva Nova | 5050400 | |
| 10% Formalin | VWR | 16004-126 | |
| 2-0 Ethibond SH | Covidien | 3369-51 | |
| 2-0 silk pops | Covidien | GS62M | |
| 2-0 silk suture with needle | DemeTECH | SK262026B0P | |
| 2-0 silk ties | DemeTECH | SK13X6620W | |
| 5 Fr micropuncture | Cook Medical | G48007 | |
| 6 Fr introducer sheath | Terumo | RSS605 | |
| 7.0 Fr Triple Lumen central venous line | Cook Medical | G47833 | |
| Aggrastat (tirofiban HCl) 2 mg | obtained from institutional pharmacy | N/A | |
| Albumin 25% 12.5 g/50 mL | obtained from institutional pharmacy | N/A | |
| Blood access sample for autologous blood recovery (spike with one-way stopcock) | Liva Nova | 7016000 | |
| Blood typing kit | Eldon Biologicals | 892165002056 | |
| Calcium gluconate 1 mg/10 mL | obtained from institutional pharmacy | N/A | |
| Calcium gluconate 1 mg/10 mL | obtained from institutional pharmacy | N/A | |
| Cefazolin 1 g | obtained from institutional pharmacy | N/A | |
| Cefazolin 1 g | obtained from institutional pharmacy | N/A | |
| Ciprofloxacin in D5W 200 mg/100 mL | obtained from institutional pharmacy | N/A | |
| Core needle biopsies 18 G TEMNO Care Fusion | Merit Medical | CA1820 | |
| CPT 8ml tubes for PBMCs | BD Bioscience | 362761 | |
| Cryogenic laster labels for frozen vials and containers - 1.28" x 0.5" | LabTAG | LCS-23 | |
| D5W 500 mL | obtained from institutional pharmacy | N/A | |
| Del Nido cardioplegia 1 L | obtained from institutional pharmacy | N/A | |
| DLP 0.64cm (1/4 in) perfusion adapter | Medtronic | 10007 | |
| Dopamine 200 mg/5 mL | obtained from institutional pharmacy | N/A | |
| Double-armed 4-0 prolene on BB needle | DemeTECH | PM1094017G0P | |
| Double-armed 4-0 prolene on RB-1 needle | DemeTECH | PM1094017C0P | |
| Echo probe covers | Microtek Medical | PC1292 | |
| EDTA 10 mL blood tubes: BD Vacutainer venous blood collection tubes BD Medical | VWR | BD-366643 | |
| Epicardial pacing wires | A&E Medical | 024-200 | |
| Epinephrine 1 mg/mL | obtained from institutional pharmacy | N/A | |
| Epinephrine 1 mg/mL | obtained from institutional pharmacy | N/A | |
| Esmolol 100 mg/10 mL | obtained from institutional pharmacy | N/A | |
| Heparin 10,000 unit/10 mL | obtained from institutional pharmacy | N/A | |
| Insulin regular (humulin R) 100 unit/1 mL | obtained from institutional pharmacy | N/A | |
| ISTAT Activated Clotting Time (ACT) Kaolin cartridges | Abbott | 03P87-25 | |
| ISTAT CG8+ cartridges | Abbott | 03P88-25 | |
| IV Amiodarone 150 mg/3 mL | obtained from institutional pharmacy | N/A | |
| IV Heparin 10,000 U/10 mL | obtained from institutional pharmacy | N/A | |
| IV Lidocaine | obtained from institutional pharmacy | N/A | |
| IV Methylprednisolone 125 mg/2 mL | obtained from institutional pharmacy | N/A | |
| Lidocaine 2% hydrochloride injection USP 100 mg/5 mL | obtained from institutional pharmacy | N/A | |
| Long 3-0 prolene on SH needle | DemeTECH | PM1093026C0P | |
| Methylprednisolone 125 mg/2 mL | obtained from institutional pharmacy | N/A | |
| Microcentrifuge tube with flat screw-cap | VWR | 16466-060 | |
| Multivitamin (infuvite adult) | obtained from institutional pharmacy | N/A | |
| Nalgene sterile specimen cryogenic vial with screw closure | VWR | 66008-740 | |
| Norepinephrine 4 mg/4 mL | obtained from institutional pharmacy | N/A | |
| OCS disposable Heart kit | TransMedics | N/A | |
| Organ Care System (OCS) Heart Module | TransMedics | N/A | |
| Oxygen tank | TransMedics | N/A | |
| Pacing cables | Remington Medical | ADAP-2000 | |
| Phenylephrine hydrochloride 100 mg/10 mL | obtained from institutional pharmacy | N/A | |
| Pledgets | Covidien | 8677-01 | |
| Pressure-volume loop catheter (Ventricath 512, 5Fr, 122 cm) | AD Instruments | Ventricath-512 | |
| Protamine 50 mg/5 mL | obtained from institutional pharmacy | N/A | |
| RNAlater | Thermo Fisher | AM7024 | |
| Scigen Tissue-Plus O.C.T compound | FisherSci | 23-730-571 | |
| Smart Perfusion Pack: double rapid prime line stock | Liva Nova | 26020000 | |
| Sodium bicarbonate 50 mEq/50 mL | obtained from institutional pharmacy | N/A | |
| Sodium bicarbonate 50 mEq/50 mL | obtained from institutional pharmacy | N/A | |
| Sterile water vial (10 mL) | obtained from institutional pharmacy | N/A | |
| Tissue-Tek Cryomold molds/adapters, Sakura Finetek | VWR | 25608-924 & 25608-916 | |
| Tissue-Tek Mega-Cassette System, Sakura Finetek | VWR | 25608-844 | |
| Umbilical tape | CP Medical | CP12A | |
| Vasopressin | obtained from institutional pharmacy | N/A | |
| Vecuronium 10 mg vial | obtained from institutional pharmacy | N/A | |
| Vessel loops | Medline | DYNJVL03 | |
| Weck Horizon Titanium Ligating Clips, Large | Teleflex | 4200 | |
| Weck Horizon Titanium Ligating Clips, Medium | Teleflex | 2200 | |
| Weck Horizon Titanium Ligating Clips, Small | Teleflex | 1201 |
References
- International Society for Heart and Lung Transplantation. , ISHLT fast facts. https://www.ishlt.org/education-and-publications/resource/ishlt-fast-facts (2024).
- Trivedi, J. R., et al. Predictors of donor heart utilization for transplantation in United States. Ann Thorac Surg. 103 (6), 1900-1906 (2017).
- Schroder, J. N., et al. Transplantation outcomes with donor hearts after circulatory death. N Engl J Med. 388 (23), 2121-2131 (2023).
- Messer, S., et al. A 5-year single-center early experience of heart transplantation from donation after circulatory-determined death donors. J Heart Lung Transplant. 39 (12), 1463-1475 (2020).
- Messer, S., et al. Outcome after heart transplantation from donation after circulatory-determined death donors. J Heart Lung Transplant. 36 (12), 1311-1318 (2017).
- Jawitz, O. K., et al. Increasing the United States heart transplant donor pool with donation after circulatory death. J Thorac Cardiovasc Surg. 159 (5), e307-e309 (2020).
- White, C. W., et al. Transplantation of hearts donated after circulatory death. Front Cardiovasc Med. 5, 8(2018).
- Lee, C., Tsai, C., Adler, E., Pretorius, V. Emerging frontier in heart transplantation: donation after circulatory death. , American College of Cardiology. https://www.acc.org/Latest-in-Cardiology/Articles/2022/11/21/13/31/Emerging-Frontier-in-Heart-Transplantation (2022).
- Messer, S., et al. A national pilot of donation after circulatory death (DCD) heart transplantation within the United Kingdom. J Heart Lung Transplant. 42 (8), 1120-1130 (2023).
- Kwon, J. H., et al. Early outcomes of heart transplantation using donation after circulatory death donors in the United States. Circ Heart Fail. 15 (12), e009844(2022).
- Pasrija, C., Tipograf, Y., Shah, A. S., Trahanas, J. M. Normothermic regional perfusion for donation after circulatory death donors. Curr Opin Organ Transplant. 28 (2), 71-75 (2023).
- Benkert, A. R., et al. heart transplant experience with normothermic regional perfusion following donation after circulatory death. JACC Heart Fail. 12 (12), 2073-2083 (2024).
- Kobashigawa, J., et al. Report from a consensus conference on primary graft dysfunction after cardiac transplantation. J Heart Lung Transplant. 33 (4), 327-340 (2014).
- Iyer, A., et al. Pathophysiological trends during withdrawal of life support: implications for organ donation after circulatory death. Transplantation. 100 (12), 2621-2629 (2016).
- White, C. W., et al. Physiologic changes in the heart following cessation of mechanical ventilation in a porcine model of donation after circulatory death: implications for cardiac transplantation. Am J Transplant. 16 (3), 783-793 (2016).
- Arnold, M., et al. Metabolic considerations in direct procurement and perfusion protocols with DCD heart transplantation. Int J Mol Sci. 25 (8), 4153(2024).
- Dann, T. M., et al. Donor heart refusal after circulatory death: an analysis of United Network for Organ Sharing refusal codes. J Thorac Cardiovasc Surg Open. 27 (18), 91-103 (2024).
- Pla, M. M., Bowles, D. E. Ex vivo gene therapy in organ transplantation: considerations and clinical translation. Hum Gene Ther. 35 (7-8), 284-297 (2024).
- Pla, M. M., et al. Ex vivo gene delivery to porcine cardiac allografts using a myocardial-enhanced adeno-associated viral vector. Hum Gene Ther. 34 (7-8), 303-313 (2023).
- Bishawi, M., et al. A normothermic ex vivo organ perfusion delivery method for cardiac transplantation gene therapy. Sci Rep. 9 (1), 8029(2019).
- Silvis, M. J. M., et al. Damage-associated molecular patterns in myocardial infarction and heart transplantation: the road to translational success. Front Immunol. 11, 599511(2020).
- Zuurbier, C. J., et al. Cardiac metabolism as a driver and therapeutic target of myocardial infarction. J Cell Mol Med. 24 (11), 5937-5954 (2020).
- Heinis, F. I., et al. Considerations for the use of porcine organ donation models in preclinical organ donor intervention research. Anim Models Exp Med. 7, 283-296 (2024).
- Hubacher, V., et al. Open- vs. closed-chest pig models of donation after circulatory death. Front Cardiovasc Med. 11, 1325160(2024).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved