Method Article
Multimodal Optical Imaging Platform for Studying Cellular Metabolism
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
Utilizing a multimodal platform combining label-free optical imaging modalities, we have developed a protocol for visualizing and quantifying cellular dynamics and metabolism. Through imaging via multiphoton fluorescence, second harmonic generation, and stimulated Raman scattering microscopy, we can generate a holistic overview of the cellular and molecular environment.
Streszczenie
Optical imaging technologies are critical in biomedical studies for their ability to obtain both morphological and functional information from biological specimens at high spatial resolution. These optical processes exploit various light-molecule interactions, such as scattering, absorption, emission, and harmonic generation, between photons and the molecules within cells, tissues, or organs. While conventional biomedical imaging has historically focused on applying a single modality, recent research has shown that these diverse techniques provide complementary insights, and their combined outputs offer a more comprehensive understanding of molecular changes in aging processes and disease development and fundamentals in cell biology.
In the past decades, label-free optical imaging methods have advanced, enabling detailed exploration of cellular and subcellular environments. For instance, multiphoton fluorescence (MPF) not only facilitates targeted protein imaging but also quantifies metabolic activity through autofluorescent coenzymes, achieving high penetration depth and spatial resolution. Second Harmonic Generation (SHG) is used to image structures like collagen in the extracellular matrix, while Stimulated Raman Scattering (SRS) maps chemical bonds and molecular composition in situ with subcellular resolution.
We have developed a multimodal imaging platform that combines MPF, SHG, and SRS modalities. The integration of these modalities into a single platform enables the acquisition of multifaceted information from the same localization within cells, tissues, organs, or even bodies, facilitating a more detailed exploration of the intricate relationships between cellular metabolism, extracellular matrix structure, and molecular composition. This multimodal system offers subcellular resolution, deep tissue penetration, in situ live-cell/tissue imaging, as well as label-free detection and instantaneous coregistration without the need for position adjustments, device switching, or postanalysis alignment. Here, we present a protocol for label-free imaging with this multimodal platform and demonstrate its application in characterizing cellular metabolism, and molecular heterogeneity in cells and tissues for studying aging and diseases.
Wprowadzenie
Optical biomedical imaging has been pivotal in advancing our understanding of biological structure and function. The images are generated by modulating excitation light and detecting signals from light-tissue interactions. The first compound microscope, developed by Hans and Zacharias Janssen around 1590, utilized two convex lenses in a tube, providing magnification up to 30x1. Modern optical microscopes, following centuries of advancements, can now achieve resolutions as fine as 1-3 nm2,3. In addition to offering high resolution, advanced imaging systems now provide deeper tissue penetration, greater efficiency, and minimal sample damage, making them especially suited for live cell and tissue imaging. Label-free imaging is particularly advantageous as it captures information without disrupting intracellular processes or compromising sample integrity.
Multiphoton fluorescence (MPF) microscopy, particularly two-photon fluorescence microscopy, has been extensively used for label-free imaging. Unlike conventional fluorescence microscopy, which relies on linear single-photon absorption and emission, MPF excitation involves the simultaneous absorption of multiple photons, whose combined energy excites a single fluorophore molecule4,5. These photons, typically in the infrared spectrum, possess half or less of the energy required for single-photon excitation. The longer wavelengths and localized excitation at the focal point in this nonlinear process result in lower scattering, deeper tissue penetration, and reduced phototoxicity.
Cellular metabolic information can be captured by label-free MPF microscopy through the detection of autofluorescence signals from endogenous metabolic substrates, such as reduced nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FAD). These coenzymes exhibit distinct excitation and emission spectra, and their fluorescence intensity ratio, known as the redox ratio (NADH/FAD), reflects the cell's oxidative state. Since Britton Chance first introduced the concept of the redox ratio in 1979, additional ratios, including NAD(P)H/FAD, NAD(P)H/(FAD + NAD(P)H), and FAD/(FAD + NAD(P)H), have been proposed6,7,8,9. Quantifying these optical redox ratios via MPF imaging provides valuable insight into metabolic dynamics. For instance, MPF imaging can distinguish cancer cells from normal cells based on their altered metabolism, demonstrating its potential for cancer diagnosis10,11,12. However, MPF-based autofluorescence detection has limitations. Other intrinsic fluorophores, such as keratin, may contribute to fluorescence intensity, leading to spectral crosstalk and inaccurate signal interpretation13. Additionally, the redox ratio only reflects overall cellular oxidation-reduction changes and does not distinguish between NADH from different sources (e.g., cytoplasmic or mitochondrial) or between NADH and NAD(P)H, as both exhibit similar spectral peaks at 450 nm, resulting in blended intensity signals14.
Second Harmonic Generation (SHG), a nonlinear optical process first demonstrated in the biomedical field in the 1980s, has been widely utilized for label-free imaging of cellular structures15,16. Similar to MPF, SHG involves the simultaneous absorption of two photons of the same energy from an ultrafast pulsed laser. These photons are recombined to emit a new photon twice the frequency of the incident light, resulting in the detection of the second-harmonic signal. This non-linear optical interaction occurs exclusively in non-centrosymmetric materials that exhibit a non-zero second-order susceptibility to induce a polarization for generating the second harmonic signal17,18. This makes SHG particularly effective for imaging the filamentous proteins and fibrillar structures, such as collagen, myosin, and tubulin, without requiring exogenous fluorescence dyes15,17,19,20. The abnormality in abundance, stiffness, alignment, and structure of fibrosis and collagen are prevalent in many conditions such as inflammation and cancer, making SHG a promising tool for efficient and non-invasive detection for certain disease conditions21,22,23. The widespread application of SHG imaging in oncological research, including studies on breast, ovarian, and skin cancers, has highlighted its crucial role in both fundamental research and potential clinical applications24,25,26,27.
Different molecules exhibit distinct vibrational energy levels, which induce varying degrees of inelastic scattering when excited by incident light-a phenomenon first characterized by C. V. Raman in 192828. The Raman effect has since been extensively utilized in optical microscopy for the detection of molecular and tissue compositions without exogenous labeling. Both Stimulated Raman Scattering (SRS) and coherent anti-Stokes Raman Scattering (CARS) excite molecular vibrations coherently and leverage the nonlinear interaction of light to produce a stronger signal compared to conventional Spontaneous Raman Spectroscopy. The SRS phenomenon was first reported in 196229. In 2008, this mechanism was integrated into three-dimensional multiphoton imaging, allowing for selective detection of chemicals based on intensity changes in the Pump and Stokes beams due to molecular vibrational transitions30. This method minimizes non-resonant background interference, generating a clean intensity signal that surpasses that of CARS. SRS imaging excels in providing multiplexed and hyperspectral imaging, enabling simultaneous detection of multiple chemical bonds and allowing high-resolution visualization of molecular composition in specimens with considerable penetration depth. Although a relatively new technique, SRS imaging has proven effective in both clinical diagnostics and metabolic research, in vivo and in vitro30,31,32,33,34,35,36. For example, SRS can differentiate brain tumor-infiltrated tissues from the cortex and white matter by quantifying the lipid-to-protein ratio, enabling the delineation of tumor margins in a label-free, non-invasive manner37,38. Additionally, metabolic alterations, often considered hallmarks of aging-related and cancer-associated diseases, can be quantitatively assessed using SRS achieved by detecting carbon-deuterium bonds in samples treated with heavy water (D2O), allowing quantitative measurement of protein synthesis, lipogenesis, and other macromolecular metabolic processes31,33,34,35,36. The ability to track metabolites with high temporal and spatial resolution positions SRS as a promising tool for disease investigation and diagnosis, with potential for broader clinical applications.
Multimodal imaging has emerged as a powerful approach in biomedical research, integrating two or more imaging modalities to gain a more comprehensive understanding of complex biological systems within the same specimen. In 2018, a label-free autofluorescence-multiharmonic (SLAM) microscopy technique was introduced, integrating two-photon fluorescence (2PF), three-photon fluorescence (3PF), SHG, and third harmonic generation (THG)39. This approach facilitates the simultaneous visualization of cellular interactions, dynamic processes, and individual components within the tumor microenvironment. SLAM microscopy offers minimal perturbation and reduced laser power requirements for the sample, enabling deep tissue profiling and providing a safer method for intravital monitoring40. Another multimodal modality, combining intrinsic fluorescence spectroscopy, diffuse reflectance spectroscopy, and Raman spectroscopy, has been developed for in situ cancer detection during surgical procedures41. Additionally, a recently designed multimodal non-linear endoscopy system, which integrates CARS, SHG, and two-photon fluorescence (TPF), has demonstrated the capability to image biological samples at sub-micron and sub-cellular spatial resolution42. Combined 2PF and SRS microscopy has similarly been utilized for high-resolution, in-vivo imaging of tissues, cells, and organelles42,43,44,45. These emerging multimodal imaging techniques harness the strengths of individual modalities, leading to improved resolution, penetration depth, and image acquisition efficiency, thus showing considerable potential for clinical and surgical applications.
This multi-modality approach is increasingly favored over single-modality imaging because it provides a broader range of measurements while mitigating the limitations associated with individual techniques. As previously discussed, MPF measures endogenous fluorescence to reflect metabolic changes, SHG can image non-centrosymmetric structures such as collagen in biological samples, and SRS predominantly detects proteins and lipids due to the high density of chemical bonds that generate distinctive Raman signals based on their vibrational modes. Given their coherent properties and the shared principle of nonlinear optical properties, these imaging modalities can be integrated into a single microscope setup utilizing ultrashort pulsed lasers, allowing for the acquisition of various biomarkers at localized regions to provide a more complete view of biological processes44,45. This paper outlines a protocol for implementing a multimodal imaging platform that integrates MPF, SHG, and SRS for biomedical research applications.
Protokół
1. Label-free multimodal imaging experiments
NOTE: This protocol focuses on the setup and acquisition procedure of the label-free multimodal imaging.
- Setup of the multimodal microscope (Figure 1A) and system calibration
- Warm up the laser and wait approximately 15-20 min.
- Power on the control units and monitors in the following sequence: Control box | Touch panel controller | AC adapter for main laser remote AC adapter for sub laser remote.
- Power on the Si photodiode detector and lock-in amplifier.
- Configure the pump laser beams and Stokes beam. Set up the laser system with a pump beam tunable from 780 nm to 990 nm, 5 - 6 ps pulse width, and 80 MHz repetition rate. The Stokes laser beam has a fixed wavelength of 1,031 nm with a 6 ps pulse and 80 MHz repetition rate. Ensure both pump and Stokes beams are at low power (at least 20 mW) to be visible on the alignment plate.
- Place one alignment plate in the optical path (right after the laser box) to check the spatial overlapping of the pumps and Stokes beams and adjust the mirror until both spots overlap at the center of the alignment plate.
NOTE: Although the pumps and Stokes beams are roughly overlapped, fine-tuning the spatial overlapping of two laser beams can achieve the optimal SRS signal. - After spatial overlapping, fine tune with the PSD (Position Sensitive Detector) by first clicking on the OPO control software. Use the hex key to carefully adjust the optical mirror 1 (OM1) and watch the PSD display for the X-Y position change to aim for the minimal deviation from the center. Then, to make more precise adjustments, use the hex key on the optical mirror 2 (OM2) until the position indicator is centered on the PSD display.
NOTE: OM1 and OM2 are two mirrors in the OPO head to adjust the laser alignment. - Finally, center the condenser by turning the two condenser-centering screws to move the iris diaphragm image to the center of the field of view.
NOTE: The field iris diaphragm can restrict the diameter of the beam of light entering the objective and thus exclude extraneous light, improving image contrast.
- Label-free multimodal imaging acquisition steps
- Apply oil to the high-numerical aperture (1.4 NA) oil condenser and mount the microscope slide onto the oiled condenser. Finally, place a large water droplet on the microscope slide for the 25x water objective. Ensure that the microscope is securely mounted and immovable and then adjust the z stage to tune the focus until the bright-field image of the biological sample can be seen under the 25x water objective.
- Begin the imaging process in the correct sequence: MPF, SHG, and SRS to avoid photobleaching, which will impact the image quality and precise analysis of MPF. To switch between MPF and SHG quickly, switch from the pump beam to the fixed Stokes beam.
NOTE: Tuning the pump laser from 800 nm (NADH and FAD autofluorescence) to 791.3 nm (CH3 Raman shift) can take 1-2 min. - Select the image resolution (512 x 512 pixels) and suitable dwell time for each modality. Use 8 µs/pixel for the MPF and SHG with average frame above 3. Use 40 µs/pixel with average frame 2 for the SRS modality.
NOTE: The average frame means the number of images to be averaged before saving. - To acquire autofluorescence with MPF, turn off the Stokes laser beam and tune the pump laser to 800 nm for exciting NADH and flavin. Install the FVOPT filter cube into the light path with 460 ± 10 nm and 515 ± 10 nm for the NADH and flavin, respectively. The power on the sample is thus approximately 15 mW.
- Acquire the collagen fiber signal using SHG. Turn off the pump laser beam, only use stokes laser beam, and set the power to be 500 mW. Only acquire the channel related to the 515 nm filter.
- Obtain the spatial distribution of proteins and lipids using SRS. Keep both laser beams on and adjust the laser beam frequency to match the specific vibrational mode for each molecule.
NOTE: Typically, 791.3 nm is used for CH3, 797 nm is used for CH2, 787 nm is for unsaturated lipids, and 794.6 is for saturated lipids. The power on the sample is thus approximately 40 mW. - To acquire the SRS hyperspectral image datasets, open the OPO control software to select sweep, set the wavelength range from 780 nm to 806.5 nm, and choose a stack number of at least 60. Then, capture the hyperspectral image stack.
NOTE: The resulting dataset contains 60 images, each representing the spatial distribution at a specific Raman shift (from 2,700 cm-1 to 3,150 cm-1). The time for hyperspectral acquisition is dependent on the field of view (FOV) of the image and scan time but will be approximately between 5 min and 8 min for a 512 x 512 image. For larger images, care should be taken to minimize the duration under the laser to prevent any photodamage, which can occur after 2 h (depending on the sample's thickness and FOV size). - Save all images of the same regions of interest (ROIs) in the same folder. The image format is Olympus .oir file.
2. Image analysis
- Use image processing software to open all saved raw images to assign the color and add the scale bar for the image display. Also use it to generate the binary mask of NADH and saturation fatty acids (SFAs, 2,880 cm-1) images for optical redox ratio and lipid unsaturation analysis.
NOTE: Make sure the mask intensity only has 0 and 1. - Use the home-built python script to do any downstream analysis (the code is available at https://github.com/lingyanshi2020/HSI_Analysis).
NOTE: This script is designed to process multiple regions of interest (ROIs) from both control and cancer samples. The script's main function requires only two inputs: the file paths for the control and cancer image folders.- Iterate through the folders, matching images with the same base name (e.g., "roi_1_flavin.tif", "roi_1_nadh.tif", "roi1_mask.tif").
- For each set of matched images, let the script perform ratiometric analysis and generate new ratiometric images based on these calculations.
The optical redox ratio is calculated as flavin / (NADH + flavin).
Lipid unsaturation is calculated as: USFAs / (SFAs + USFAs) where USFAs represent unsaturated fatty acids. - All ratiometric values from the images are then stored in a pandas DataFrame for easy manipulation. Perform one-way ANOVA and use matplotlib with styple "ggplot" or seaborn to do the barplot or boxplot for quantification.
- Use the 2,930 cm-1 (CH3) and 2,850 cm-1 (CH2) images to generate a subtracted (CH3 - CH2) image. Then, blend CH2 image CH3 - CH2 image in RGB color space with a custom LUT that mimics H&E staining to generate the digital histology image46.
- After acquisition of hyperspectral SRS images, employ the home-built python script to do the k-means clustering to segment the images based on spectral similarities (the code is available at https://github.com/lingyanshi2020/HSI_Analysis).
- Load the .tiff image stacks with the tifffile package. Reshape the SRS hyperspectral image stack (x, y, spectra) into a 2D array (pixels, spectra).
- Use the scikit-learn package to deploy k-means algorithm to this 2D array, with the defined number of clusters.
- Reshape the resulting cluster labels back into the original image dimensions, creating a segmentation map.
- Assign unique colors to each cluster, generating a false-color image that represents different biochemical compositions within the tissue.
- Use matplotlib to plot the spectra of each cluster with mean value (solid line) and standard deviation (shadow area)
- Follow the protocol of the penalized reference matching SRS (PRM-SRS) protocol using MATLAB to create the spatial label-free lipid subtype detection with SRS hyperspectral image datasets47.
- Organize the images and save them in a 300 dpi .tif format.
Wyniki
The images in Figure 2 are representative of the results obtained from following the protocol for acquiring autofluorescence of FAD and NADH as well as the four SRS channels for protein, total lipids, unsaturated lipids, and saturated lipids. Here we also generate a pseudo-histological image as described in the protocol through RGB color blending. The acquisition of the MPF and SRS channels provides the image files that will be later utilized in ratiometric analysis. An example of this analysis can be seen in Figure 3 on human lung tissue. Following image acquisition in the protocol, our image analysis methodology utilizing Python or ImageJ leverages the ratio of distinct channels to provide quantitative metabolic information. As shown in the lipid unsaturation and optical redox ratio images of Figure 3, the ratiometric analysis provides a color map of the distribution of relative metabolic activity and molecular composition. We have leveraged these measurements to make observations regarding the changes in metabolic pathways and lipid content of specific tissue, pathologies, or distinct biological factors. As seen in Figure 4, this can provide a quantitative comparison of healthy and tumor tissue by comparing the average values of oxidative stress and lipid unsaturation in addition to its distribution in a 2D microscopy image.
For ratiometric analysis of MPF and SRS images, we commonly target the optical redox and unsaturation ratio, as shown by Eq (1) and (2).
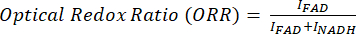 (1)
(1)
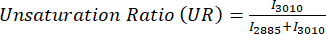 (2)
(2)
Where IC is the intensity of pixels from channel C. Thus, we measure the ratio per pixel and acquire the spatial distribution of these metabolic and molecular markers.
In addition to the ratiometric analysis, Figure 4 also demonstrates another potential avenue of application for our multimodal platform: hyperspectral image analysis. As described in this protocol, we can acquire an SRS hyperspectral image by performing a sweep across multiple laser wavelengths by utilizing a tunable pump beam. This allows us to reconstruct the CH region of the Raman shift spectrum for each pixel within the microscopy image, joining both the chemical information provided by Raman spectroscopy and the spatial information afforded by optical microscopy. In the workflow for image analysis, we highlight two techniques implemented for analyzing these hyperspectral images: PRM-SRS for biomolecule detection and k-means clustering. Figure 4E displays the application of PRM-SRS on an image of a mouse hepatic tumor. The PRM-SRS algorithm provides the correlation between the spectra for each individual pixel, adjusted by a penalty for shifts in the Raman spectrum; the algorithm then generates probability distribution images for each of the lipids.
K-means clustering of the pixel spectra is another technique we implement to visualize the grouping of specific spectral phenotypes, enabling separation by bond concentration and molecular composition through intensity and spectral shape respectively. Figure 5 provides an example of how image analysis via k-means clustering can be applied in our protocol. We expect k-mean clusters to align structurally with features from the pseudo-histology figures in addition to providing further clustering from features that are not as easily discerned from either the SRS channels or the pseudo-Hematoxylin and Eosin histological staining (H&E). PRM-SRS and k-means clustering analysis complement this label-free imaging platform by providing chemical and semi-targetable information regarding specific analytes and molecular bonds without the need for exogenous probing.
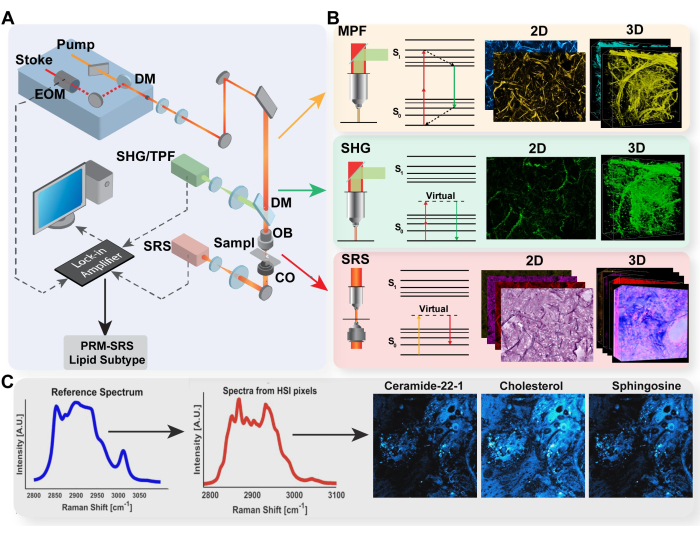
Figure 1: Diagram of multimodal imaging platform and PRM-SRS biomolecule detection. (A) Diagram of hardware set-up for MPF/TPF, SHG, and SRS imaging system. (B) Light path and Jablonski diagram for TPF, SHG, and SRS with representative acquired images for each modality. (C) PRM-SRS workflow diagram for spectral reference matching between spontaneous Raman spectroscopy and SRS hyperspectral imaging. Abbreviations: MPF = multiphoton fluorescence; TPF = two-photon fluorescence; SHG = second harmonic generation; SRS = stimulated Raman scattering; PRM = penalized reference matching. Please click here to view a larger version of this figure.
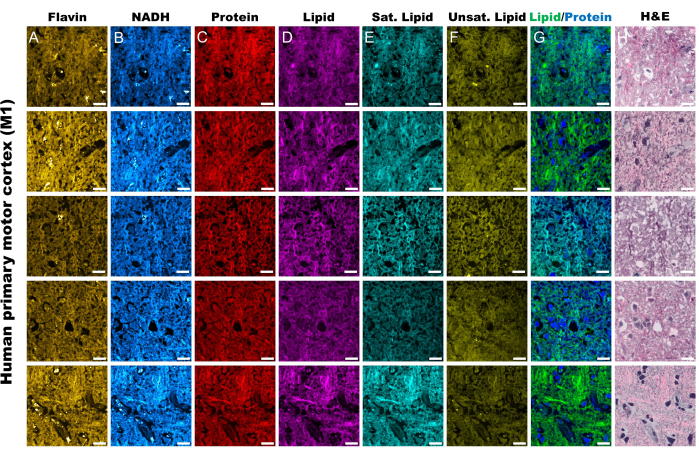
Figure 2: MPF and SRS channels from human primary motor cortex tissue (M1). (A,B) MPF autofluorescence channels for Flavin/FAD and NADH. (C-F) SRS channels captured at specific Raman shift peaks; the CH3 asymmetric stretching peak (2,930 cm-1) for proteins, the CH2 asymmetric stretching peak (2,845 cm-1) for lipids, the 2,885 cm-1 peak for saturated fatty acids, and 3,010 cm-1 peak for unsaturated fatty acids. (G) Merged SRS protein (blue) and lipid (green) channels to outline their respective spatial distribution. (H) SRS pseudo-histological images (SRH) mirroring H&E staining. Scale bars = 20 µm. Abbreviations: MPF = multiphoton fluorescence; FAD = flavin adenine dinucleotide; NADH = reduced nicotinamide adenine dinucleotide; SRS = stimulated Raman scattering; SRH = SRS histology. Please click here to view a larger version of this figure.
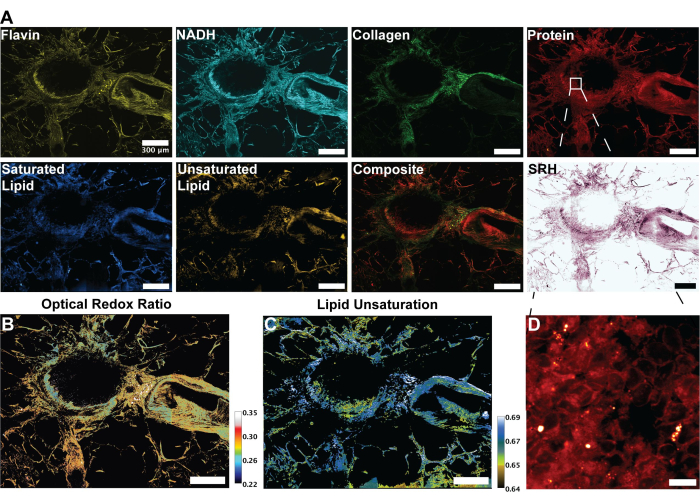
Figure 3: MPF, SHG, and SRS images and analysis of human lung tissue. (A) MPF autofluorescence for Flavin/FAD and NADH, SHG signal for collagen fibers, SRS signal for protein (2,930 cm-1), lipid (2,845 cm-1), saturated lipids (2,885 cm-1) and unsaturated lipids (3,010 cm-1) peaks, and a composite merged image of the multiple modalities.(B) Ratiometric image of optical redox ratio. (C) Ratiometric image of lipid unsaturation. (D) Zoomed-in image of epithelial cells in the inner layer of bronchi. Scale bars = 200 µm. Abbreviations: MPF = multiphoton fluorescence; SHG = second harmonic generation; SRS = stimulated Raman scattering; FAD = flavin adenine dinucleotide; NADH = reduced nicotinamide adenine dinucleotide. Please click here to view a larger version of this figure.
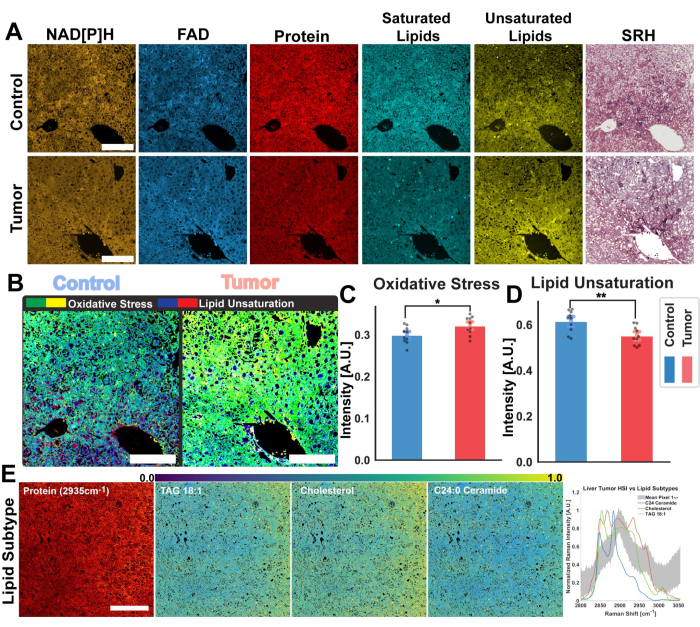
Figure 4: Ratiometric and Hyperspectral analysis of mouse hepatic tumors. (A) MPF autofluorescence images for NAD[P]H and FAD, SRS channels for total protein, saturated, and unsaturated lipid, and pseudo-histological images for healthy control mouse liver (top) and tumorous (bottom) tissue. (B) Oxidative stress (yellow-green), as measured by the optical redox ratio, and lipid unsaturation (blue-red) calculated from the control (left) and tumor (right) tissues. Scale bars = 100 µm. (C,D) Bar graphs demonstrating ratiometric differences in oxidative stress and lipid unsaturation between control (blue) and tumor (red) liver samples. Significance statistics: * for a significance of p ≤ 0.05, ** for a significance of p ≤ 0.01. (E) PRM-SRS lipid subtype analysis results from hyperspectral images. From left to right: SRS protein channel for structural reference, scale bar = 100 µm, probability distribution image of lipid subtypes (TAG 18:1, Cholesterol, and C24:0 Ceramide). Graph of lipid subtype reference spectra and mean pixel spectra from SRS HSI across the CH region. Abbreviations: MPF = multiphoton fluorescence; SRS = stimulated Raman scattering; NAD[P]H = reduced nicotinamide adenine dinucleotide phosphate; FAD = flavin adenine dinucleotide; HSI = hyperspectral images. Please click here to view a larger version of this figure.
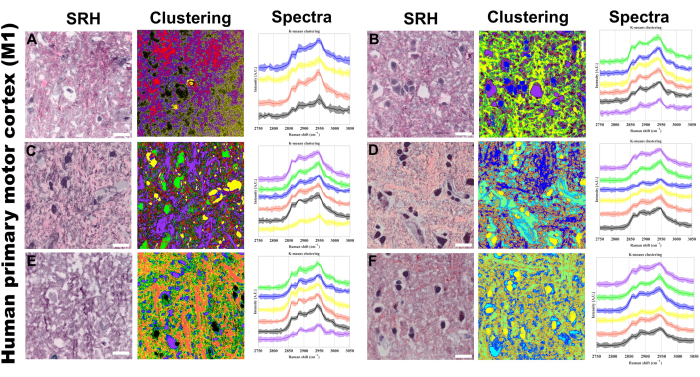
Figure 5: SRS Hyperspectral k-means clustering on human primary motor cortex (M1). (A-F) Six representative regions showing: left: Stimulated Raman Histology (SRH) images providing H&E-like tissue visualization; middle: corresponding k-means clustering results; Right: cluster-specific Raman spectral profiles with mean intensities (solid lines) and standard deviations (shaded areas). Number of centroids ranging from 4 to 6 means. Unsupervised clustering was used, so different colors in the clustered images represent distinct chemical compositions identified by the k-means algorithm. Each cluster (represented by a unique color) corresponds to regions with similar spectral profiles in the CH stretching region. Scale bar = 20 µm. Abbreviations: SRH = SRS histology. Please click here to view a larger version of this figure.
Dyskusje
This multimodal system is a powerful imaging platform for capturing a holistic visualization of the molecular environment of samples across a wide range of biological origins and pathological conditions. The advantage of leveraging different label-free modalities lies in the capacity to acquire complementary information and target specific analytes that might otherwise be difficult or impossible in a single label-free imaging technique. Specifically, the three nonlinear imaging techniques (SRS, MPF, SHG) mentioned in this paper allow for the quantification of macromolecule composition, energy dynamics via the optical redox ratio, and structural information, including extracellular matrix composition and morphology6,48,49. Furthermore, compared to utilizing each single imaging modality separately, imaging with one combined microscope system allows for immediate image registration and shorter sample storage times. We have even conducted live cell imaging with this approach, possibly due to the lower photoexposure compared to confocal fluorescence microscopy, using select time points to acquire each modality sequentially as stipulated in the above protocol. Aside from their individual advantages, the multimodal platform also benefits from the multiple perspectives on biological condition through the label-free measurement of different biomarkers.
Label-free imaging of autofluorescent proteins via MPF has primarily been directed towards the quantification of the optical redox ratio, a measure of redox reactions facilitated by the oxidation of FAD into FAD+ and reduction of NAD+ into NADH50. This is a significant marker of metabolism within cells and tissues as it provides a measurement of the relative activity between oxidative phosphorylation and glycolysis, the two main pathways for ATP generation6. Specifically, decreases in the NADH concentration and increases in FAD+ are a marker for increased oxidative phosphorylation in mitochondria; meanwhile, the reverse is true for increased glycolysis in the cytoplasm. The tendency to prefer one metabolic pathway over the other in energy production has been linked to several pathological changes and pathway activations linked to cancer, highlighting the potential use of the ORR as an early detection marker6,51.
Similarly, the acquisition of SHG fibrous collagen signal leverages a nonlinear label-free imaging modality to quantify and visualize a biological marker for health. SHG can effectively track the distribution of Type I-III collagen due to their non-centrosymmetric structure. Fibrous collagen proteins measured by SHG have been recognized as important diagnostic markers for several diseases including cancer and fibrosis25,26,27,52,53. In addition to its detection as a marker for disease, our acquisition of collagen via SHG can serve as a strong indicator for structure in cells and tissues due to the role of fibrous collagen in the composition of the extracellular matrix and structural boundaries across tissues54,55. Through the SHG collagen signal, we can then make informed observations of separate cells or distinct tissue functional units without the need for an exogenous marker for cellular membranes. Overall, SHG quantification of collagen fibrils presents a clear benefit to both diagnostic ability and ease of analysis when integrated with other label-free imaging techniques.
Utilizing SRS microscopy, we can capture several markers for macromolecule composition, particularly for lipids and fatty acids. In this protocol, we describe how to acquire the SRS signal for unsaturated and saturated fatty acids for ratiometric analysis of lipid unsaturation within the same sample. An upright laser-scanning microscope with a 25x water objective was applied for near-IR throughput. The laser beams passed through the sample and were collected by a high-numerical-aperture oil condenser (1.4 NA). A high O.D. shortpass filter (950 nm) was used to block the Stokes beam while only allowing the pump beam to reach a Si photodiode detector to detect the stimulated Raman loss signal. The output current from the photodiode was terminated and filtered. Subsequently, a lock-in amplifier at 20 MHz demodulated the current in X with a zero-phase shift, and a software module used the demodulated signal to generate the image during laser scanning.
Studies have shown that changes in lipid unsaturation have a profound effect on the cellular and organelle membrane and a dysregulation in lipid metabolism can be a strong marker for several diseases, including cancer and neurodegenerative diseases56,57,58,59. For a more comprehensive overview of lipid dysregulation, we analyze subtype composition using SRS hyperspectral imaging. SRS hyperspectral images (HSIs) combine the high-resolution spatial information acquired by SRS microscopy with the chemically significant signal of Raman spectroscopy through sequential tuning as described in the protocol. These pixel spectra are then analyzed in two methods, clustering via k-means and biomolecule detection with PRM-SRS.
K-means clustering of the pixel spectra results in the separation of regions within a 2D image by molecular concentration and composition due to the linear relationship between SRS signal intensity and molecular bond concentration60,61. Through this methodology, we apply the protocol to ascertain regions that possess specific molecular phenotypes as prescribed by the pixel spectra and shared spectral centroid. For a thorough examination of the distinct molecular expression in specific pixels, we implement PRM-SRS to perform spectral matching between a reference spectrum for a specific molecule captured with spontaneous Raman spectroscopy and the pixel spectra from SRS HSI acquisition. In doing so, we can determine the probability of expression for distinct molecules without utilizing exogenous probes or labels. This analysis has demonstrated the ability to track certain lipid subtypes across tissues and biological models, and the capacity to identify pathological changes in lipid composition changes and thereby dyslipidemia47. Furthermore, recent advances in improving spectral resolution through spectral splitting of picosecond pulse propagation through silica fibers further incentivizes the application of SRS HSI as a modality for label-free biochemical visualization62. Through these methods highlighted in the protocol, we establish a platform to perform comprehensive ratiometric and spectral analysis utilizing a label-free SRS modality.
In addition to the relative complexity and novelty of these techniques, there are some important limitations in our approach to consider. Acquiring MPF images with a picosecond pulsed laser, as opposed to the commonly utilized femtosecond lasers, requires higher fluorophore excitation power and risks photobleaching. With that in mind, we have optimized the above protocol to mitigate the potential photodamage from our platform by reducing the laser power incident on the specimen. Furthermore, while previous studies have demonstrated the capability of evaluating the optical redox ratio from MPF autofluorescence imaging of NADH and FAD, fluorescence lifetime imaging microscopy (FLIM) has demonstrated higher accuracy in evaluating energy metabolism63,64,65. This is due to the ability to differentiate protein-bound and unbound concentrations of NADH and FAD based on their distinct fluorescence lifetimes, Compared to the capabilities of MPF, this has allowed FLIM to be used for comprehensive studies of metabolic activity from distinct energy pathways, including oxidative phosphorylation and glycolysis16,63,64,65,66,67,68. While this protocol excludes FLIM, we expect the modality to be integrated into this imaging platform in the near future, enhancing the energy metabolism analysis we currently perform. Nevertheless, with the protocol established here, we present a workflow and hardware setup for a multimodal imaging approach that can measure biological structure metabolism from multiple perspectives.
Ujawnienia
The authors have no conflicts of interest to declare.
Podziękowania
We thank Dr. Gloria Pryhuber and her HuBMAP team members for providing human lung tissue slices. We thank Dr. Kun Zhang for providing human brain tissues. We also thank Dr. Gen-Sheng Feng for providing mouse liver samples. We acknowledge support from NIHU54DK134301, NIH R01GM149976, NIH U01AI167892, NIH R01HL170107, NIH 5R01NS111039, NIH R21NS125395, NIH U54CA132378, UCSD Startup funds, Sloan Research Fellow Award, and CZI DAF2023-328667 Award.
Materiały
| Name | Company | Catalog Number | Comments |
| 460 nm Filter Cube | Olympus | OCT-ET 460/50M32 | |
| Bandpass Filter | KR Electronics | KR2724 | 8 MHz |
| BNC 50 Ohm Terminator | Mini Circuits | STRM-50 | |
| DC power supply | TopWard | 6302D | |
| Dichroic Mount | Thorlabs | KM100CL | |
| FIJI ImageJ | ImageJ | ||
| High NA oil condenser | Olympus | 6-U130 | |
| High O.D. shortpass filter | Thorlabs | 950 nm | |
| Inverted Laser Scanning Microscope | Olympus | FV1200MPE | |
| Lock-in Amplifier | Zurich Instruments | ||
| picoEmerald Laser System | Applied Physics & Electronics, Inc. | Synchronized pulsed pump beam (tunable 720–990 nm wavelength, 5–6 ps pulse width, and 80 MHz repetition rate) and Stokes (wavelength at 1032 nm, 6 ps pulse width, and 80 MHz repetition rate) | |
| SI Photodiode Detector | Home Built | ||
| Touch Panel Controller | Olympus | ||
| VF-300 Compresstome | Precisionary |
Odniesienia
- Wollman, A. J. M., Nudd, R., Hedlund, E. G., Leake, M. C. From Animaculum to single molecules: 300 years of the light microscope. Open Biol. 5 (4), 150019(2015).
- Vicidomini, G., Bianchini, P., Diaspro, A. STED super-resolved microscopy. Nat Methods. 15 (3), 173-182 (2018).
- Weber, M., et al. MINSTED fluorescence localization and nanoscopy. Nat Photonics. 15 (5), 361-366 (2021).
- Denk, W., Strickler, J. H., Webb, W. W. Two-photon laser scanning fluorescence microscopy. Science. , (1990).
- Hell, S. W., et al. Three-photon excitation in fluorescence microscopy. J Biomed Optics. 1 (1), 71-74 (1996).
- Alhallak, K., Rebello, L. G., Muldoon, T. J., Quinn, K. P., Rajaram, N. Optical redox ratio identifies metastatic potential-dependent changes in breast cancer cell metabolism. Biomed Opt Express. 7 (11), 4364-4374 (2016).
- Pena, A. -M., et al. Multiphoton FLIM imaging of NADH and FAD to analyze cellular metabolic activity of reconstructed human skin in response to UVA light. Multiphoton Microscopy in the Biomedical Sciences XIX. 10882, 23-33 (2019).
- Hu, L., Wang, N., Cardona, E., Walsh, A. J. Fluorescence intensity and lifetime redox ratios detect metabolic perturbations in T cells. Biomed Opt Express. 11 (10), 5674-5688 (2020).
- Chance, B., Schoener, B., Oshino, R., Itshak, F., Nakase, Y. Oxidation-reduction ratio studies of mitochondria in freeze-trapped samples. NADH and flavoprotein fluorescence signals. J Biol Chem. 254 (11), 4764-4771 (1979).
- Ostrander, J. H., et al. Optical redox ratio differentiates breast cancer cell lines based on estrogen receptor status. Cancer Res. 70 (11), (2010).
- Skala, M. C., et al. In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia. Proc Natl Acad Sci USA. 104 (49), 19494-19499 (2007).
- Varone, A., et al. Endogenous two-photon fluorescence imaging elucidates metabolic changes related to enhanced glycolysis and glutamine consumption in precancerous epithelial tissues. Cancer Res. 74 (11), 3067-3075 (2014).
- Malak, M., James, J., Grantham, J., Ericson, M. B. Contribution of autofluorescence from intracellular proteins in multiphoton fluorescence lifetime imaging. Sci Rep. 12, 16584(2022).
- Blacker, T. S., Duchen, M. R. Investigating mitochondrial redox state using NADH and NADPH autofluorescence. Free Radic Biol Med. 100, 53-65 (2016).
- Roth, S., Freund, I. Second harmonic generation in collagen. J Chem Phys. 70 (4), 1637-1643 (1979).
- Freund, I., Deutsch, M. Second-harmonic microscopy of biological tissue. Opt Lett. 11 (2), 94(1986).
- Boyd, R. W., Gaeta, A. L., Giese, E. Nonlinear optics. Springer Handbook of Atomic, Molecular, and Optical Physics. Drake, G. W. F. , Springer. 1097-1110 (2023).
- Aghigh, A., et al. Second harmonic generation microscopy: a powerful tool for bio-imaging. Biophys Rev. 15 (1), 43-70 (2023).
- Mohler, W., Millard, A. C., Campagnola, P. J. Second harmonic generation imaging of endogenous structural proteins. Methods. 29 (1), 97-109 (2003).
- Cox, G. Biological applications of second harmonic imaging. Biophys Rev. 3 (3), 131(2011).
- Brown, E., et al. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat Med. 9 (6), 796-800 (2003).
- Keikhosravi, A., Bredfeldt, J. S., Sagar, A. K., Eliceiri, K. W. Chapter 28 - Second-harmonic generation imaging of cancer. Methods Cell Bio. 123, 531-546 (2014).
- Hui Mingalone, C. K., et al. Bioluminescence and second harmonic generation imaging reveal dynamic changes in the inflammatory and collagen landscape in early osteoarthritis. Lab Invest. 98 (5), 656-669 (2018).
- Tsafas, V., et al. Polarization-dependent second-harmonic generation for collagen-based differentiation of breast cancer samples. J Biophotonics. 13 (10), e202000180(2020).
- Nadiarnykh, O., LaComb, R. B., Brewer, M. A., Campagnola, P. J. Alterations of the extracellular matrix in ovarian cancer studied by Second Harmonic Generation imaging microscopy. BMC Cancer. 10, 94(2010).
- Xiong, S. Y., Yang, J. G., Zhuang, J. Nonlinear spectral imaging of human normal skin, basal cell carcinoma and squamous cell carcinoma based on two-photon excited fluorescence and second-harmonic generation. Laser Phys. 21 (10), 1844-1849 (2011).
- Tilbury, K., Campagnola, P. J. Applications of second-harmonic generation imaging microscopy in ovarian and breast cancer. Perspect Medicin Chem. 7, 21-32 (2015).
- Raman, C. V., Krishnan, K. S. A New type of secondary radiation. Nature. 121 (3048), 501-502 (1928).
- Eckhardt, G., et al. Stimulated Raman scattering from organic liquids. Phys Rev Lett. 9 (11), 455-457 (1962).
- Freudiger, C. W., et al. Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science. 322 (5909), 1857-1861 (2008).
- Li, Y., et al. Microglial lipid droplet accumulation in tauopathy brain is regulated by neuronal AMPK. Cell Metab. 36 (6), 1351-1370.e8 (2024).
- Lu, F. -K., et al. Label-free neurosurgical pathology with stimulated Raman imaging. Cancer Res. 76 (12), 3451-3462 (2016).
- Shi, L., et al. Optical imaging of metabolic dynamics in animals. Nat Commun. 9 (1), 2995(2018).
- Zhang, L., et al. Spectral tracing of deuterium for imaging glucose metabolism. Nat Biomed Eng. 3 (5), 402-413 (2019).
- Li, Y., et al. Bioorthogonal stimulated Raman scattering imaging uncovers lipid metabolic dynamics in Drosophila brain during aging. GEN Biotechnol. 2 (3), 247-261 (2023).
- Li, X., et al. Quantitative imaging of lipid synthesis and lipolysis dynamics in Caenorhabditis elegans by stimulated Raman scattering microscopy. Anal Chem. 91 (3), 2279-2287 (2019).
- Ji, M., et al. Detection of human brain tumor infiltration with quantitative stimulated Raman scattering microscopy. Sci Transl Med. 7 (309), 309ra163(2015).
- Ji, M., et al. Rapid, label-free detection of brain tumors with stimulated Raman scattering microscopy. Sci Transl Med. 5 (201ra119), 201ra119(2013).
- You, S., et al. Intravital imaging by simultaneous label-free autofluorescence-multiharmonic microscopy. Nat Commun. 9, 2125(2018).
- You, S., et al. Label-free deep profiling of the tumor microenvironment. Cancer Res. 81 (9), 2534-2544 (2021).
- Jermyn, M., et al. Highly accurate detection of cancer in situ with intraoperative, label-free, multimodal optical spectroscopy. Cancer Res. 77 (14), 3942-3950 (2017).
- Pshenay-Severin, E., et al. Multimodal nonlinear endomicroscopic imaging probe using a double-core double-clad fiber and focus-combining micro-optical concept. Light Sci Appl. 10 (1), 207(2021).
- Wu, W., Li, X., Qu, J. Y., He, S. In vivo imaging of biological tissues with combined two-photon fluorescence and stimulated Raman scattering microscopy. J Vis Exp. (178), e63411(2021).
- Li, X., Jiang, M., Lam, J. W. Y., Tang, B. Z., Qu, J. Y. Mitochondrial imaging with combined fluorescence and stimulated Raman scattering microscopy using a probe of the aggregation-induced emission characteristic. J Am Chem Soc. 139 (47), 17022-17030 (2017).
- Li, Z., et al. Multimodal imaging of metabolic activities for distinguishing subtypes of breast cancer. Biomed Opt Express. 14 (11), 5764-5780 (2023).
- Fung, A. A., et al. Label-free optical biopsy reveals biomolecular and morphological features of diabetic kidney tissue in 2D and 3D. , (2024).
- Zhang, W., et al. Multi-molecular hyperspectral PRM-SRS microscopy. Nat Commun. 15 (1), 1599(2024).
- Xu, F. X., et al. Discrimination of lipid composition and cellular localization in human liver tissues by stimulated Raman scattering microscopy. J Biomed Opt. 29 (1), 016008(2024).
- Zoumi, A., Yeh, A., Tromberg, B. J. Imaging cells and extracellular matrix in vivo by using second-harmonic generation and two-photon excited fluorescence. Proc Natl Acad Sci USA. 99 (17), 11014-11019 (2002).
- Liaudanskaya, V., et al. Mitochondria dysregulation contributes to secondary neurodegeneration progression post-contusion injury in human 3D in vitro triculture brain tissue model. Cell Death Dis. 14 (8), 1-15 (2023).
- Walsh, A. J., et al. Optical metabolic imaging identifies glycolytic levels, sub-types and early treatment response in breast cancer. Cancer Res. 73 (20), 6164-6174 (2013).
- Conklin, M. W., et al. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol. 178 (3), 1221-1232 (2011).
- Sun, W., et al. Nonlinear optical microscopy: use of second harmonic generation and two-photon microscopy for automated quantitative liver fibrosis studies. J Biomed Optics. 13 (6), 064010(2008).
- Nejim, Z., Navarro, L., Morin, C., Badel, P. Quantitative analysis of second harmonic generated images of collagen fibers: a review. Res Biomed Eng. 39 (1), 273-295 (2023).
- Burke, K., Brown, E. The use of second harmonic generation to image the extracellular matrix during tumor progression. Intravital. 3 (3), e984509(2015).
- Balasubramanian, P., Prabhakaran, M. P., Sireesha, M., Ramakrishna, S. Collagen in human tissues: Structure, function, and biomedical implications from a tissue engineering perspective. Polymer Composites - Polyolefin Fractionation - Polymeric Peptidomimetics - Collagens. Abe, A., Kausch, H. H., Möller, M., Pasch, H. 251, 173-206 (2012).
- Dadsena, S., et al. Lipid unsaturation promotes BAX and BAK pore activity during apoptosis. Nat Commun. 15, 4700(2024).
- Barelli, H., Antonny, B. Lipid unsaturation and organelle dynamics. Curr Opin Cell Biol. 41, 25-32 (2016).
- Harayama, T., Antonny, B. Beyond fluidity: The role of lipid unsaturation in membrane function. Cold Spring Harb Perspect Biol. 15 (7), a041409(2023).
- Yoon, H., Shaw, J. L., Haigis, M. C., Greka, A. Lipid metabolism in sickness and in health: Emerging regulators of lipotoxicity. Mol Cell. 81 (18), 3708-3730 (2021).
- Villazon, J., Shi, L. Utilizing K-means clustering on hyperspectral DO-SRS images of the mouse hippocampus. CLEO 2024, Technical Digest Series. , Optica Publishing Group. (2024).
- Fu, D., et al. Quantitative chemical imaging with multiplex stimulated Raman scattering microscopy. J Am Chem Soc. 134 (8), 3623-3626 (2012).
- Periasamy, A., Diaspro, A. Special section guest editorial: Multiphoton microscopy. J Biomed Optics. 8 (3), 327(2003).
- Lakowicz, J. R., Szmacinski, H., Nowaczyk, K., Johnson, M. L. Fluorescence lifetime imaging of free and protein-bound NADH. Proc Natl Acad Sci USA. 89 (4), 1271-1275 (1992).
- Blacker, T. S., et al. Separating NADH and NADPH fluorescence in live cells and tissues using FLIM. Nat Commun. 5, 3936(2014).
- Xiong, H., et al. Background-free imaging of chemical bonds by a simple and robust frequency-modulated stimulated Raman scattering microscopy. Opt Express. 28 (10), 15663-15677 (2020).
- Georgakoudi, I., Quinn, K. P. Label-free optical metabolic imaging in cells and tissues. Annu Rev Biomed Eng. 25, 413-443 (2023).
- Alam, S. R., Wallrabe, H., Christopher, K. G., Siller, K. H., Periasamy, A. Characterization of mitochondrial dysfunction due to laser damage by 2-photon FLIM microscopy. Sci Rep. 12 (1), 11938(2022).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone