Method Article
Spatula Montevideo Device for the Vitrification of Mammalian Embryos
In This Article
Summary
Here, we present the Spatula Montevideo protocol for vitrifying mouse and sheep pre-implantation embryos, consisting of a homemade spatula and utilizing homemade solutions. Embryos are rapidly equilibrated in two cryoprotectant solutions of increasing concentration, loaded onto the spatula, immersed in liquid nitrogen, and finally sealed with a 0.5 mL straw.
Abstract
This study describes an efficient and cost-effective vitrification method for mouse and sheep embryo cryopreservation known as the Spatula Montevideo (MVD). This protocol utilizes a homemade spatula as the carrier device, which is prepared with a gel-loader tip. Embryos are rapidly equilibrated in two homemade cryoprotectant solutions of increasing concentration, loaded into the spatula, and then directly immersed in liquid nitrogen. Approximately 30-40 pre-implantation stage mouse embryos can be loaded on the spatula, which is subsequently sealed with a 0.5 mL straw. The use of spatulas instead of cryotubes, which are frequently used to store vitrified embryos, optimizes space in the liquid nitrogen dewars. Additionally, the warming process requires only a homemade sucrose solution of decreasing concentration to rehydrate the embryos and restore their morphology. Our laboratory has successfully used the Spatula Montevideo for mouse and sheep pre-implantation embryos for over 10 years. The Spatula Montevideo has demonstrated an average recovery rate (recovered/vitrified embryos) of 84% and a survival rate (viable/recovered embryos) of 97% in mouse embryos. Vitrification of murine embryos has yielded better results than slow freezing, although no differences in reproductive outcomes (pregnancy, delivery, and birth rates) have been observed. In sheep embryos, similar survival and embryo development rates have been achieved with Spatula Montevideo compared to a commercial device. Although the pregnancy rate was higher for the commercial device, the lamb survival rate was not significantly different among the devices. The Spatula Montevideo vitrification method is a straightforward cryopreservation technique that can be used to store surplus embryos, back up genetically modified mouse lines and sheep models, and facilitate their exchange among researchers worldwide.
Introduction
The efficiency of genetically modified (GM) animal generation has significantly increased due to the development of endonuclease-based methods, particularly CRISPR/Cas9. Many laboratories worldwide produce various GM mouse and rat lines that should be cryopreserved to prevent genetic drift and maintain the integrity of the original phenotype1. Additionally, archiving eliminates the need for constant breeding, which often results in excessive animal numbers. Some laboratories send GM mouse lines as cryopreserved embryos or sperm to international repositories, facilitating the exchange of these models while avoiding the transport of live animals. This ensures accessibility to lines without the repetitive generation of identical GM animal models2. For these reasons, embryo and sperm cryopreservation are paramount in any GM animal facility. Generally, embryo cryopreservation is preferred for storing GM lines because embryos contain the complete diploid genome with modifications and can be easily recovered via embryo transfer.
Vitrification is a non-equilibrium process characterized by an imbalance between water entering the cell and water forming extracellular crystals around it. This method prevents the formation of intracellular ice crystals, distinguishing it from the slow freezing method (SLF)3 (see Figure 1). The high concentration of cryoprotectants and direct contact with liquid nitrogen (LN2) enable ultra-rapid cooling (~40 °C/s), achieving a vitreous state of the samples within seconds. Moreover, vitrification is faster, easier, and less expensive than slow freezing4. It requires minimal LN2 and typically employs simple equipment that can be commercially purchased or homemade5 (see Figure 1).
Additionally, the warming process is generally fast and replicable. It should be performed quickly to avoid cryoprotectant toxicity and to maintain the vitreous state3. When freezing embryos, many laboratories consider the convenience of the carrier device, its reliability, robustness, holding capacity, and costs.
We prefer the minimum volume spatula vitrification method for routine cryopreservation of sheep and mouse embryos, using the Spatula Montevideo (MVD) device6. Initially, we employed the slow freezing method7 but quickly transitioned to vitrification. To create a simpler and more space-efficient device for the LN2 dewar, we modified the homemade spatula from Tsang and Chow (2009)8, which is plugged into a cryotube, to develop the Spatula MVD which is sealed with a 0.5 mL straw (see Table of Materials). The spatula features a gel-loader tip (see Table of Materials), where a platform of approximately 1 mm² is formed by gently melting the tip with fine forceps, creating a petal-like plate for loading the embryos. This platform can accommodate up to 40 mouse embryos in a small vitrification drop. An identification plug rod (see Table of Materials) is inserted into the open end of the gel-loader tip.
The aim of this method is to vitrify embryos in a simple, fast, and inexpensive method that, in addition, saves space in the nitrogen dewar. This system has been successfully used for many years, providing excellent embryo survival rates (~97%) and reproductive outcomes, particularly in mouse embryos. Since Spatula MVD is prepared in the laboratory, it is a low-cost method compared to commercial devices9. Moreover, it saves space in the nitrogen dewar in comparison to the use of cryotubes8,10.
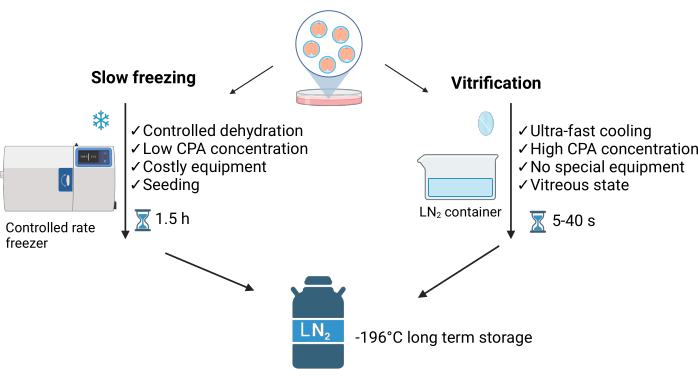
Figure 1: Comparison of the main two techniques used for embryo cryopreservation. This figure has been modified with permission from Crispo et al.1. Created in BioRender.com. Please click here to view a larger version of this figure.
Protocol
Animals used to obtain mouse embryos were housed and handled according to national law 18.611 and international animal care guidelines11. Experimental protocol (permit number #007-18) was opportunely approved by the Institut Pasteur de Montevideo Animal Care and Use Committee (written consent was given). Experimental protocols for sheep oocytes/embryos were approved by the Institutional Animal Care Committee Fundación IRAUy (protocol #001-2017), complying with the ARRIVE guidelines12 and were carried out in accordance with international animal care guidelines11.
1. Vitrification (Figure 2)
- Spatula MVD preparation
- Shorten the gel-loader tip (see Table of Materials) by cutting off the first 10 mm and the tip cone.
NOTE: This creates a shorter, straight tip, facilitating the final sealing with the 0.5 mL straw (see Table of Materials). - Hold the end of the gel-loader tip with watchmaker's #5 forceps (see Table of Materials) approximately 1.0 cm from the tip. Gently place the tip into the flame of a Bunsen burner (see Table of Materials) and hold for 8-10 s to form a petal-like platform of approximately 1 mm2.
- Remove from the flame and hold tightly with the forceps for 5 s.
- Using a stereomicroscope (see Table of Materials), check that the petal-like platform (~ 1 mm2) is formed and the distal edge is sealed.
NOTE: The platform must be closed at the distal end to prevent LN2 infiltration during vitrification. Ensure the platform is clean and free of plastic threads. If it is too small or has threads, trim the tip and repeat the procedure. - Repeat steps 1.1.1-1.1.4 to prepare the necessary number of spatulas (1 spatula = up to 40 mouse embryos).
- Sterilize the spatulas using ultraviolet (UV) rays or ethylene oxide and store them in a sterile tube or box.
- On the day of vitrification, retrieve the spatulas, handling them from the opposite end of the platform. Insert an identification rod (see Table of Materials) into each spatula and place them on the stereomicroscope base. Label the rod with a permanent marker or liquid nitrogen-resistant sticker label.
NOTE: Always avoid touching the platform when handling the spatulas. The total length of the complete device (spatula + 0.5 mL straw + identification rod) should not exceed more than 1 cm of the length of the goblet.
- Shorten the gel-loader tip (see Table of Materials) by cutting off the first 10 mm and the tip cone.
- Preparation of vitrification solutions
- Prepare the FS solution: Dissolve 1.5 g of density gradient medium (see Table of Materials) and 0.85 g of sucrose (see Table of Materials) in 5 mL of phosphate-buffered saline (PBS; see Table of Materials). Heat in a boiling water bath until completely dissolved, then sterilize using a 0.22 µm filter (see Table of Materials).
NOTE: This solution can be stored at 4 °C for up to 6 months. - Prepare the pre-vitrification solution (PV): On the day of vitrification, mix 10% ethylene glycol (see Table of Materials), 10% dimethyl sulfoxide (see Table of Materials), and 80% M2 medium (see Table of Materials). A total volume of 100 µL is needed for 5 spatulas.
- Prepare the vitrification solution (V): On the day of vitrification, combine 15% ethylene glycol, 15% dimethyl sulfoxide, 10% M2 medium, and 60% FS solution. A total volume of 100 µL is required for 5 spatulas.
- Prepare the FS solution: Dissolve 1.5 g of density gradient medium (see Table of Materials) and 0.85 g of sucrose (see Table of Materials) in 5 mL of phosphate-buffered saline (PBS; see Table of Materials). Heat in a boiling water bath until completely dissolved, then sterilize using a 0.22 µm filter (see Table of Materials).
- Embryo dehydration and vitrification process
- Prepare LN2: Pour LN2 into a suitable container (e.g., a 10 cm x 30 cm x 20 cm insulated polystyrene container) and place as many 0.5 mL straws as spatulas to be used.
NOTE: To facilitate visualization of the spatula platform during manipulation under LN2, it is suggested that a colored bottom-insulated polystyrene container be used to create a contrast effect.
CAUTION: Wear appropriate goggles and gloves (see Table of Materials) when handling LN2. - Prepare solutions: On a 100 mm plastic Petri dish (see Table of Materials), place the following drops: 100 µL of M2, 20 µL of PV solution, and 20 µL of V solution for each spatula.
- Vitrify embryos: Place the embryos to be vitrified in the M2 drop. Handle 30-40 embryos at a time, incubating them in the PV solution drop for 30 s, and then in the V solution drop for another 30 s at room temperature (RT).
- Use a pulled glass capillary (see Table of Materials) with an aspirator tube assembly (see Table of Materials). Pre-load the capillary with at least 1 µL of the respective solution before placing the embryos.
NOTE: Embryos will be at different heights in the drops, so adjust the stereomicroscope micrometer to locate them. Retrieve embryos from each drop when 10 s remains. All embryo manipulations must be conducted under the stereomicroscope. - Load embryos: Aspirate the embryos using minimal V solution (e.g., ~ 0.1 µL) and place them quickly onto the spatula platform.
- Immersion in LN2: After loading the embryos, immediately immerse the spatula platform in LN2 for 5 s (holding the spatula by hand, by its rod). Then, while maintaining the spatula in LN2 vapors, seal it with a 0.5 mL straw using long stainless-steel tweezers (see Table of Materials).
NOTE: Remove LN2 from the straw before sealing to prevent expansion during warming. Ensure the spatula and straw are aligned properly to avoid contact between the embryo drop and straw wall. For convenience, sealed spatulas can remain in the LN2 container until all spatulas are loaded with embryos, refilling with LN2 as necessary. - Repeat procedure: Repeat steps 1.3.3 to 1.3.7 for each group of embryos, using a fresh pair of PV and V drops.
- Storage: Transfer the spatulas to goblets (see Table of Materials) and then to a LN2 storage dewar (see Table of Materials).
- Prepare LN2: Pour LN2 into a suitable container (e.g., a 10 cm x 30 cm x 20 cm insulated polystyrene container) and place as many 0.5 mL straws as spatulas to be used.

Figure 2: Scheme of the vitrification process using Spatula MVD. See the text for more details. Created in BioRender. Meikle, M. (2025) https://BioRender.com/x96y902. Please click here to view a larger version of this figure.
2. Warming (Figure 3)
- Solution preparation
- Prepare a 1 M sucrose solution dissolving 0.85 g of sucrose in 2.5 mL of M2 medium. Sterilize the solution with a 0.22 µm filter.
NOTE: This solution can be prepared and stored at 4 °C for up to 1 week. - Prepare 0.5 M and 0.25 M sucrose solutions by diluting the 1 M sucrose solution with the appropriate volume of M2 medium.
- Maintain the sucrose solutions at 37 °C on a hot plate (see Tables of Materials) until the warming starts.
- Prepare a 1 M sucrose solution dissolving 0.85 g of sucrose in 2.5 mL of M2 medium. Sterilize the solution with a 0.22 µm filter.
- Warming plate preparation
- On a 100 mm plastic Petri dish, place the following solution drops: a 500 µL drop of 0.5 M sucrose, a 50 µL drop of 0.5 M sucrose, and a 50 µL drop of 0.25 M sucrose for each spatula to be warmed. Place three drops of M2 medium (100 µL each) for washing.
- Warming process
NOTE: The warming process takes place at RT, e.g., 20-22 °C on the base of a stereomicroscope.- Retrieve the sealed spatulas from the dewar. Place them in an appropriate container with LN2. See Figure 3.
- Place the warming plate on the stereomicroscope. One at a time, hold the base of the spatula with stainless-steel tweezers, and with a gloved hand, pull out the identification rod and then the straw. Quickly dip the platform containing the embryos into the 0.5 M sucrose drop of 500 µL.
- Ensure that all embryos have fallen into the drop; if necessary, gently move the spatula to assist their release. Load the embryos into a pulled glass capillary, place them in the 50 µL drop of 0.5 M sucrose solution, and incubate for 2 min.
- Place the embryos in the 50 µL drop of 0.25 M sucrose solution and incubate for another 2 min.
NOTE: Before placing the embryos in each solution, pre-load the capillary with the solution to be used. - Finally, wash the embryos three times in different drops of M2 medium to remove the sucrose.
- Incubate the embryos under conditions appropriate for each species for at least 1 h before use.
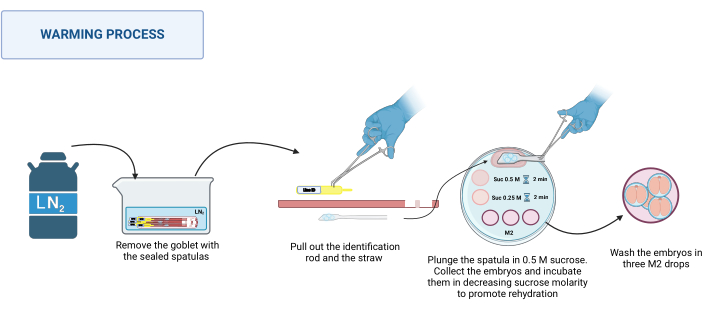
Figure 3: Scheme of the Spatula MVD warming process. See the text for more details. Created in BioRender. Meikle, M. (2025) https://BioRender.com/k91k191. Please click here to view a larger version of this figure.
Results
We initially used the traditional slow-freezing method based on propylene glycol solutions, straws, and controlled rate freezing equipment7. However, after considering the vitrification method a potentially easier and more cost-effective option, we conducted a study to compare the outcomes of traditional slow freezing (SLF) vs. Spatula MVD in 8-cell in vivo derived and 2-cell in vitro produced mouse embryos13 (Table 1 and Table 2). Results are presented in terms of "recovery rate" which defines the number of embryos recovered from the device after warming over the total number of cryopreserved embryos. "Survival rate" refers to the number of viable embryos over the recovered embryos from the device. Embryos were considered viable if blastomeres were intact (two blastomeres in 2-cell embryos and at least four blastomeres in 8-cell embryos)14. These terms are used to assess whether the device and the whole process are suitable for cryopreservation.
A significantly higher survival rate (Table 1 and Table 2) and development rate at 3.5 days post coitum (dpc; e.g., morulae and blastocyst embryos, Table 1) were achieved for Spatula MVD than for the SLF group in mouse embryos. Although both methods reached an acceptable hatching rate, it was significantly lower than the control fresh group, as expected (non-vitrified embryos; Table 1). Vitrified/warmed and control fresh 2-cell embryos were transferred to recipient females, revealing no differences both in pregnancy rate (pregnant/total transferred females) and birth rate (pups born/transferred embryos in total recipient females; Table 2). These outcomes lead us to switch from the SLF method to vitrification.
| Nº of embryos | Survival rate at 2.5 dpc (viable/recovered embryos from the device) | Embryo development rate at 3.5 dpc (morulae-blastocysts/viable embryos) | Hatching rate at 5.5 dpc (hatched blastocysts/cultured embryos) | |
| Slow freezing | 410 | 89.3 % (366/410)a | 91.8 % (336/366)a | 49.7 % (90/181)a |
| Spatula MVD | 341 | 92.7 % (316/341)b | 97.2 % (307/316)b | 41.0 % (68/166)a |
| Fresh embryos | 458 | - | 98.7 % (452/458)b | 67.8 % (139/205)b |
Table 1: Survival and in vitro development rates of 8-cell murine embryos subjected to vitrification or slow freezing. In vivo 8-cell murine embryos were cryopreserved by Spatula MVD or slow freezing and compared to fresh embryos. Fresh embryos were collected from females at 2.5 dpc and cultured in the same conditions as the other groups. The survival rate (viable/recovered embryos from the device) was determined after warming/thawing. Viable embryos were in vitro cultured. Embryo development rate (morulae-blastocysts/viable embryos) and hatching rate (hatched/cultured embryos) were determined at 3.5 dpc and 5.5 dpc, respectively. Different superscripts indicate significant differences (P < 0.05).
| Nº of embryos | Recovery rate (recovered from the device/cryopreserved embryos) | Survival rate (viable/recovered embryos from the device) | Pregnancy rate (pregnant/transferred females) | Birth rate in total transferred females (pups born/transferred embryos) | |
| Slow freezing | 531 | 88.3 % (469/531)a | 85.1 % (399/469)a | - | - |
| Spatula MVD | 431 | 84.2 % (363/431)a | 94.7 % (344/363)b | 53.3 % (8/15)a | 17.8 % (47/264)a |
| Fresh embryos | 446 | - | 97.7 % (436/446)b | 53.3 % (8/15)a | 20.6 % (53/257)a |
Table 2: Recovery and survival rates of 2-cell murine embryos subjected to vitrification or slow freezing. Reproductive outcomes of vitrified/warmed versus fresh embryos.In vitro-produced 2-cell embryos were cryopreserved by Spatula MVD or SLF. Recovery (recovered from the device/cryopreserved embryos) and survival rate (viable/recovered embryos from the device) were determined after warming or thawing. Viable warmed and fresh embryos were transferred to recipient females (15 females per group, ~17 embryos per female). Pregnancy (pregnant/transferred females) and birth (pups born/transferred embryos) rates were determined. Different superscripts indicate significant differences (P < 0.05).
Spatula MVD was also tested for vitrification of in vitro produced sheep pre-implantation embryos and compared with a commercial device6. Results are shown in Table 3. The quality of embryos was assessed by morphology following the criteria recommended by the International Embryo Technology Society (IETS)15, and those excellent and good Grade 1 embryos were allotted to each experimental group.
Neither the survival rate (~70% and ~16%, 3 h or 24 h after warming, respectively) nor the embryo hatching on day 8 was influenced by the vitrification method. The percentage of blastocysts on day 8 was lower for both vitrification techniques compared to the control group (fresh embryos) (~ 8% vs 20.5%, respectively; P < 0.05). These results suggest that in vitro produced sheep embryos can also be vitrified using the Spatula MVD. In addition, previous results were complemented by transferring sheep embryos subjected to slow freezing, Spatula MVD, or a commercial device to recipient females16. Results are shown in Table 4. Embryo survival rate (viable embryos on Day 30/transferred embryos), pregnancy rate (pregnant/transferred ewes), fetal loss from gestation to birth (lambs born assessed at birth/viable embryos on Day 30), and lamb survival rate (live lambs 1 week after birth/lambs born) were evaluated between experimental groups.
Results showed that embryo survival rate after embryo transfer was similar for SLF (26.5%) and Spatula MVD (22.2%) but was higher for the commercial device group (52.0%, P < 0.05). Reproductive outcomes (gestation length, fetal loss, birth weight, and lamb survival rates) were not significantly affected by the cryopreservation method. Overall, Spatula MVD is also a suitable method for the vitrification of ovine pre-implantation embryos.
| N° of embryos | Survival rate % (viable/cryopreserved embryos) | Development rate % (morulae-blastocysts at Day 6/cleaved embryos) | Hatching rate % (hatched blastocysts/cleaved embryos) | ||
| 3 h | 24 h | ||||
| Spatula MVD | 165 | 69.6 ± 2.4a | 14.6 ± 3.5a | 11.1 ± 2.1b | 6.4 ± 1.9b |
| Commercial device | 165 | 71.3 ± 1.3a | 17.7 ± 4.5a | 12.7 ± 3.2b | 10.2 ± 2.9b |
| Control group (fresh embryos) | 408 | - | - | 41.3 ± 3.7a | 20.5 ± 4.5a |
Table 3: Survival and in vitro development rates of ovine embryos subjected to vitrification by Spatula MVD or a commercial device.In vitro produced 2-8 cell stage embryos (Day 2 after fertilization) were vitrified using Spatula MVD or a commercial device and then warmed. Survival rate (viable/cryopreserved) was analyzed at 3 h and 24 h post-warming. The development and hatching rates were defined as the number of morulae and blastocysts obtained on Day 6 and the number of hatched embryos, respectively, over the number of cleaved (2-8 cells) embryos. Different superscripts in the same column indicate significant differences (P < 0.05). Data is shown as Mean ± SEM.
| Embryo survival (viable embryos on Day 30/transferred embryos) | Pregnancy rate (pregnant/transferred ewes) | Fetal loss (lambs born accessed at birth/viable embryos on Day 30) | Gestation length (d) | Birth weight (g) | Lamb survival rate (live lambs one week after birth/lambs born) | |
| Spatula MVD | 22.2 %a (28/126) | 32.9 %a (25/76) | 4.0 %a (1/25) | 150.7 ± 1.8a | 4102 ± 112.4a | 87.5 %a (21/24) |
| Commercial device | 52.0 %b (91/175) | 66.0 %b (62/94) | 11.0 %a (8/73) | 150.4 ± 1.9a | 3980 ± 221.2a | 83.1 %a (54/65) |
| Slow freezing | 26.5 %a (36/136) | 38.3 %a (28/73) | 6.7 %a (2/30) | 150.4 ± 2.1a | 4319 ± 107.3a | 89.3 %a (25/28) |
Table 4: Effect of the cryopreservation method on embryo survival after transfer, pregnancy maintenance, and lamb production. Morulae to expanded blastocysts of in vivo or in vitro produced embryos were subjected to vitrification/slow freezing and warming/thawing and were transferred to multiparous Merino synchronized ewes. Embryo survival rate (viable embryos on Day 30/transferred embryos), pregnancy rate (pregnant/transferred ewes), fetal loss from gestation to birth (lambs born accessed at birth/viable embryos on Day 30), gestation length (d), birth weight (g) and lamb survival rate (live lambs one week after birth/lambs born) were compared among experimental groups.
Moreover, genetically modified mouse lines no longer in use in our facility were backed up as two-cell embryos using the Spatula MVD method. A minimum of 300 two-cell embryos, produced mainly by in vitro fertilization (or natural mating), were vitrified for each mouse line. Quality control of the process was performed by warming two or three spatulas of each line and transferring the embryos to pseudopregnant female recipients. Representative results of different genetically modified mouse lines are shown in Table 5. On average, the recovery rate was 90.4%, and the survival rate was 96.8%. The pregnancy rate was 80%, and the birth rate was 31.5% on average. Of note, the birth rate showed a wide range of results from 12% to 45%, which could be due to the different genetic modifications that can affect embryo sensitivity and embryo transfer outcomes. In general, these results were consistent with those previously obtained in the facility.
| Mouse line | No. of vitrified/warmed embryos | Recovery rate (recovered from the device/ cryopreserved embryos) | Survival rate (viable/recovered embryos from the device) | Pregnancy rate (pregnant/transferred females) | Birth rate (pups born/transferred embryos) |
| 1 | 60 | 83.3 % (50/60) | 96.0 % (48/50) | 50 % (1/2) | 19.4 % (7/36) |
| 2 | 35 | 100 % (35/35) | 100 % (35/35) | 100 % (1/1) | 39.1 % (9/23) |
| 3 | 43 | 76.7 % (33/43) | 84.8 % (28/33) | 100 % (1/1) | 38.8 % (7/18) |
| 4 | 41 | 92.6 % (38/41) | 97.3 % (37/38) | 100 % (2/2) | 31.4 % (11/35) |
| 5 | 66 | 98.4 % (65/66) | 100 % (65/65) | 100 % (3/3) | 36.6 % (22/60) |
| 6 | 70 | 94.2 %(66/70) | 95.4 % (63/66) | 33 % (1/3) | 12 % (6/50) |
| 7 | 74 | 87.8 % (65/74) | 100 % (65/65) | 100 % (3/3) | 45 % (27/60) |
| Average | 90.4 % (352/389) | 96.8 % (341/352) | 80 % (12/15) | 31.5 % (89/282) |
Table 5: Representative results of the quality control vitrification process performed in 2-cell embryos of different genetically modified mouse lines. GM mouse lines were backed up as two-cell embryos (minimum 300 embryos) using the Spatula MVD method. Quality control of the process was performed by warming two or three spatulas of each line and transferring the embryos to pseudopregnant female recipients. Recovery (recovered from the device/cryopreserved embryos) and survival rate (viable/recovered embryos from the device) were determined after warming. Viable warmed embryos were transferred in 2-cell stage to recipient females (1 to 3 females per line, ~18 embryos per female). Pregnancy (pregnant/transferred females) and birth (pups born/transferred embryos) rates were determined.
Finally, we recently performed a comparative work in which fresh and vitrified-warmed mouse zygotes were microinjected with CRISPR reagents and then in vitro cultured or transferred to pseudopregnant females17. The objective was to evaluate whether vitrified zygotes -using Spatula MVD- could be a suitable source of embryos for microinjection in B6D2F1/J and C57BL/6J zygotes. The recovery and survival rates were similar to the previous results obtained for 2-8 cell stage embryos (Table 6). Overall, vitrified-warmed zygotes survived microinjection, developed into hatched blastocysts or produced live pups, and demonstrated mutations induced by the CRISPR/Cas9 system. However, the mutation rate was significantly lower in vitrified zygotes than in fresh ones. Zygote cryobanking seems to be an alternative source of embryos to perform microinjection sessions on demand, avoiding the breeding and maintenance of animals throughout the entire year and enhancing the implementation of 3Rs in CRISPR technology.
| Strain zygote | Nº of cryopreserved zygotes | Recovery rate (recovered from the device /cryopreserved embryos) | Survival rate (viable/recovered embryos from the device) |
| B6D2F1/J | 380 | 97.1 % (369/380) | 90.8 % (335/369) |
| C57BL/6J | 800 | 84.0 % (672/800) | 92.3 % (620/672) |
Table 6: Recovery and survival rates of vitrified-warmed B6D2F1/J and C57BL/6J zygotes. In vivo-produced zygotes were collected from B6D2F1/J and C57BL/6J donor females, vitrified by Spatula MVD, and warmed. Recovery (recovered from the device/cryopreserved embryos) and survival (viable/recovered embryos from the device) rates were determined after warming.
Discussion
The Spatula MVD is an easy-to-assemble, low-cost, minimum-volume vitrification device with high embryo holding capacity and optimal liquid nitrogen dewar occupancy. This method has proven to be simple, fast, and robust. On average, we have achieved not only high embryo recovery and survival rates of vitrified embryos (~90%) but also good reproductive outcomes when performing embryo transfer in mice (~80% pregnancy rate and ~30% birth rate), which are comparable to other minimum volume vitrification methods18,19,20.
Critical steps in the Spatula MVD vitrification method include: (i) Spatula preparation. This technique requires considerable expertise. The platform produced in the gel loader tip by heating with forceps should be ~ 1 mm2 and must be closed at the end. If the tip remains open, bubbles may form when plunging the spatula in the sucrose drop during the warming process because of temperature differences. Bubbles interfere with embryo searching and recovery. (ii) Embryo incubation in vitrification solutions. Because of high density, the embryos stay at different heights in the drops. A fast aspiration of all the embryos is needed. Toxicity is expected if embryos remain in these solutions for longer periods at room temperature. (iii) Spatula vitrification drop. The volume of the vitrification drop -containing the embryos- in the platform should be ~0.5-1.0 µL; larger cryoprotectant volumes affect embryo viability. We recommend using pulled capillaries with an inner diameter a little bit wider than the size of an embryo, which would carry the volume needed. Additionally, bigger drops in the platform may increase the possibility of getting stuck in the 0.5 mL straw inside the wall when closing the system. (iv) Spatula sealing. Special care must be taken when closing the spatula with the 0.5 mL straw; both elements have to be aligned. Embryo drops in the spatula must not be disturbed. (v) During warming, follow the order of removal of the rod identifier and the 0.5 mL straw. Otherwise, the identifier may be expelled by the temperature difference.
This technique requires operator training in different stages. The main troubleshooting could be the recovery of the embryos from the spatula. If less than 70-80% of the vitrified embryos are recovered when warming the spatula, this may be due to (i) incomplete loading of the embryos onto the spatula platform or (ii) embryos being retained in the 0.5 mL straw inner wall when the system is closed. To overcome the first issue, the loading of the drop containing the embryos must be performed under the stereomicroscope using an aspirator tube assembly and a pulled capillary loaded with the cryoprotectant media containing the embryos and 2-3 little air bubbles along the capillary, which allows a correct flow of the liquid. For the second issue, If no embryos are visible in the platform during warming, cut the cotton plug to open the 0.5 mL straw and wash it twice with 500 µL of 0.5 M sucrose in M2 (see section 2, step 1), expelling the solution in a dish for embryo recovery.
The limitations of the method are mainly related to the critical stages already mentioned. We believe that the key point is the spatula preparation. It takes training to perform a good spatula, but once learned, it can be mastered. Since the media are homemade, this method cannot be used in human reproduction clinics.
The main advantage of the Spatula MVD is that it is a low-cost device compared to commercial ones9. Furthermore, it saves space in the liquid nitrogen dewar compared to cryotube vitrification8. We have tested its functionality on mouse and sheep embryos at different stages. It is particularly simpler, faster, more economical, and more effective than the slow freezing method.
This method can be used to vitrify embryos of different non-human mammalian species in a wide range of research areas.
Disclosures
The authors declare no conflicts of interest.
Acknowledgements
We thank Alejo Menchaca for his contribution to the development of the Spatula MVD. GS received funding from the Biotechnology Postgraduate Program - Facultad de Ciencias - Universidad de la República (https://webmasterbiotecnol.wixsite.com/biotec1) (Grant Number: GS 2437 347). GS, MNM, and MC received funding from FOCEM - Fondo para la Convergencia Estructural del Mercosur (https://focem.mercosur.int/es/) (Grant Number: COF 03/11). GS and MC are fellows of Sistema Nacional de Investigadores, Agencia Nacional de Investigación e Innovación, Uruguay. (https://www.anii.org.uy/). The funders (Biotechnology Postgraduate Program - Facultad de Ciencias -Universidad de la República; FOCEM - Fondo para la Convergencia Estructural del Mercosur; Sistema Nacional de Investigadores, Agencia Nacional de Investigación e Innovación, Uruguay) had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| 0.22 µm filter | Sartorius | 16534 | Minisart syringe filter 0.2 µL |
| 0.5 mL straw | Minitube | 13408/0044 | French type medium paillette |
| Aspirator tube assembly | Sigma | A5177 | |
| Bunsen burner | Indulab | 7000134 | |
| Dimethyl sulfoxide | Sigma | D2650 | |
| Ethylene glycol | Sigma | 102466 | |
| Ficoll PM70 | Sigma | F2878 | Density gradient medium |
| Gel-loader tip | Eppendorf | 10411193 | |
| Glass capillary | Marienfeld | 2900000 | Microhaematocrit capillary tube |
| Gloves for LN2 handling | Tempshield | CE0338 | Cryogloves |
| Goblets | Minitube | 16913/1133 | |
| Goggles for LN2 handling | 3M | 40661-00000-10 | Anti-fog, anti-scratch coating |
| Hot plate | Labec | LA-SH-II-5B | Digital model |
| Identification rod | IMV technologies | 6326 | Non steril LG48 mm |
| Insulated styrofoam container | |||
| LN2 storage dewar | Taylor-Wharton | XT21-AI | Cryoscience |
| Long stainless-steel twezeers | Fine Science Tools | 11000-25 | |
| M2 | Sigma | M7167 | |
| PBS | Home-made | Phosphate Buffered saline. Usually 1 L of concentrated 5x buffer is prepared and then diluted to 1x. Recipe: 40 g sodium chloride, 1 g potassium chloride, 7.2 g anhydrous disodium phosphate, 1.2 g monopotassium phosphate. Add distilled water and sterilize by autoclave. | |
| Plastic Petri dish | Falcon | 351029 | Not TC-treated Bacteriological Petri Dish |
| Stereomicroscope | Olympus | SZ2-LGB | |
| Sucrose | Sigma | S1888 | |
| Watchmaker’s #5 forceps | Fine Science Tools | 11254-20 |
References
- Crispo, M., Meikle, M. N., Rülicke, T. Cryopreservation of Valuable Mouse and Rat Lines. Rodent Quality Control: Genes and Bugs. Laboratory Animal Science and Medicine. , Springer. Cham. (2024).
- Lloyd, K., Franklin, C., Lutz, C., Magnuson, T. Reproducibility: use mouse biobanks or lose them. Nature. 522 (7555), 151-153 (2015).
- Hart-Johnson, S., Mankelow, K. Archiving genetically altered animals: a review of cryopreservation and recovery methods for genome-edited animals. Lab Anim. 56 (1), 26-34 (2022).
- Shaw, J. M., Jones, G. M. Terminology associated with vitrification and other cryopreservation procedures for oocytes and embryos. Hum Reprod Update. 9 (6), 583-605 (2003).
- Arav, A. Cryopreservation of oocytes and embryos. Theriogenology. 81 (1), 96-102 (2014).
- dos Santos Neto, P. C., et al. Cryotolerance of Day 2 or Day 6 in vitro produced ovine embryos after vitrification by Cryotop or Spatula methods. Cryobiology. 70 (1), 17-22 (2015).
- Renard, J. P., Babinet, C. High survival of mouse embryos after rapid freezing and thawing inside plastic straws with 1-2 propanediol as cryoprotectant. J Exp Zool. 230 (3), 443-448 (1984).
- Tsang, W. H., Chow, K. L. Mouse embryo cryopreservation utilizing a novel high-capacity vitrification spatula. Biotechniques. 46 (7), 550-552 (2009).
- Kuwayama, M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 67 (1), 73-80 (2007).
- Nakao, K., Nakagata, N., Katsuki, M. Simple and efficient vitrification procedure for cryopreservation of mouse embryos. Exp Anim. 46 (3), 231-234 (1997).
- National Research Council. Guide for the Care and Use of Laboratory Animals. , National Academies Press. Washington, DC. (1996).
- du Sert, N. P., et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 18 (7), e3000410(2020).
- Meikle, M. N., Schlapp, G., Menchaca, A., Crispo, M. Minimum volume Spatula MVD vitrification method improves embryo survival compared to traditional slow freezing, both for in vivo and in vitro produced mice embryos. Cryobiology. 84, 77-81 (2018).
- Momozawa, K., et al. Efficient vitrification of mouse embryos using the Kitasato Vitrification System as a novel vitrification device. Reprod Biol Endocrinol. 15 (1), 29(2017).
- Stringfellow, D. A., Givens, M. D. Manual of the International Embryo Transfer Society: A Procedural Guide and General Information for the use of Embryo Transfer Technology Emphasizing Sanitary Procedures. , International Embryo Transfer Society. (2010).
- Dos Santos-Neto, P. C., Cuadro, F., Barrera, N., Crispo, M., Menchaca, A. Embryo survival and birth rate after minimum volume vitrification or slow freezing of in vivo and in vitro produced ovine embryos. Cryobiology. 78, 8-14 (2017).
- Schlapp, G., Meikle, M. N., Pórfido, J. L., Menchaca, A., Crispo, M. Zygote cryobanking applied to CRISPR/Cas9 microinjection in mice. PLoS One. 19 (7), e0306617(2024).
- Kamoshita, M., et al. Recent advances of oocyte/embryo vitrification in mammals from rodents and large animals. Anim Sci J. 95 (1), e13931(2024).
- Qiu, J., et al. Equilibrium vitrification of mouse embryos using low concentrations of cryoprotectants. Cryobiology. 98, 127-133 (2021).
- Tsang, W. H., Chow, K. L. Cryopreservation of mammalian embryos: Advancement of putting life on hold. Birth Defects Res C Embryo Today. 90 (3), 163-175 (2010).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved