Method Article
Network Pharmacology and Validation of the Antidepressant Mechanisms of Qiangzhifang in a Chronic Restraint Stress-induced Depression Rat Model
* These authors contributed equally
In This Article
Summary
This study evaluated the antidepressant efficacy of Qiangzhifang in a rat model of chronic restraint stress-induced depression and elucidated its regulatory effect on HIF-1 and JAK-STAT pathways by network pharmacology and molecular docking analysis.
Abstract
Depression is a complex psychiatric disorder that poses significant treatment challenges.Qiangzhifang (QZF), a compound used in traditional Chinese medicine, demonstrates potential clinical efficacy in treating depression.However, the mechanisms of action and active ingredients of QZF have not been fully elucidated.The primary aim of this study was to elucidate the effective active ingredients and potential molecular mechanisms of QZF for the alleviation of depression by integrating network pharmacology predictions with experimental validations.
We adopted a chronic restraint stress (CRS) rat model and conducted behavioral tests such as the open field test (OFT), sucrose preference test (SPT), and forced swimming test (FST) to evaluate the therapeutic effects of QZF on depression. Regarding behavioral parameters, the QZF group exhibited significantly higher body mass, sucrose preference ratio, and central zone residence time compared to the model group (P < 0.01, P < 0.01, P < 0.01), and a significantly reduced immobilization time in the forced swimming test (P < 0.001).Network pharmacology and molecular docking studies suggest that QZF may have antidepressant effects by modulating the HIF-1 and JAK-STAT pathways, with key target genes including AKT1, IL-6, MTOR, and TP53, implicated in inflammation, neuroprotection, and apoptosis.In conclusion, this study offers new insights into the modernization and development of Chinese medicine compounds for the comprehensive treatment of depression.
Introduction
Depression, a pervasive global health challenge, is characterized by a persistent low mood, reduced interest and pleasure, and cognitive and neurological impairments1. As reported by the World Health Organization, depression impacts approximately 380 million people worldwide, and this figure is expected to increase2. As a complex, multifactorial mental disorder, depression affects patients' quality of life and poses a considerable economic and medical burden on society, characterized by high incidence, recurrence rates, and disability rates3.
The etiology of depression is complex, with the precise mechanisms not yet fully understood. As research in this field progresses, factors such as neuroinflammation, oxidative stress, and apoptosis have garnered significant attention. Studies indicate that patients with depression exhibit elevated levels of pro-inflammatory cytokines like TNF and interleukin-1β compared to healthy individuals, and a higher prevalence of depression is observed in those with inflammatory conditions4. In oxidative stress, reactive oxygen species (ROS) are overproduced in response to harmful stimuli, overwhelming the body's antioxidant defenses and leading to an imbalance between oxidative and antioxidant systems, thereby causing tissue damage. Elevated oxidative stress in depression can enhance lipid peroxidation and exacerbate damage to cellular genes and proteins, impacting neuronal function and contributing to neuronal degeneration, apoptosis, and impaired plasticity5. Additionally, the alterations observed in clinical presentations, biochemical markers, and brain structures in patients with depression are linked to apoptosis. Imaging studies reveal reduced hippocampal volume and atrophy in patients with depression, with neuronal apoptosis potentially playing a pivotal role in these changes6.
Currently, drug treatment is the primary approach for managing depression, with selective serotonin reuptake inhibitors (SSRIs) and norepinephrine reuptake inhibitors (NRIs) being frequently employed in clinical practice7. However, these drugs are accompanied by significant adverse effects. In addition to central nervous system symptoms like headache and insomnia, most antidepressants also commonly exhibit gastrointestinal side effects, including nausea and diarrhea8,9. Some antidepressants can also cause sexual dysfunction10, which severely impacts treatment outcomes and reduces medication adherence among patients with depression11. Moreover, the efficacy of these drugs is limited for some patients. Recent metabolomics studies have indicated that individual differences in gut microbiota may influence drug efficacy12. Therefore, the development of safer and more effective treatments remains a critical focus in depression research.
Traditional Chinese medicine (TCM) formulations have demonstrated significant potential in treating depression, attributed to their synergistic effects involving multiple components, targets, and pathways13. TCM posits that vigorous Yang qi is essential for maintaining the body's vitality. Therefore, Professor Yuanqing Ding, leveraging the unique principles of TCM diagnosis and treatment and extensive clinical experience, proposed that "yang yu shen tui" is the fundamental pathogenesis of depression. Based on this concept, he developed Qiangzhifang (QZF) to specifically address this pathogenesis14. The clinical application of QZF in treating depression has demonstrated significant efficacy, with a total effective rate of 71.43%15. QZF is composed of various traditional Chinese medicinal materials, including Ramulus cinnamomi (gui zhi, GZ), Polygala tenuifolia (yuan zhi, YZ), Alpinia oxyphylla miq (yi zhi ren, YZR), Paeonia lactiflora (bai shao, BS), Fritillariae cirrhosae bulbus (chuan bei mu, CBM), Panax ginseng (ren shen, RS), Rhodiola rosea L (hong jing tian, HJT), and licorice (gan cao, GC) (Supplemental File 1). Studies have shown that Polygala tenuifolia is rich in saponins andexhibits neuroprotective effects16. Similarly, the Ramulus Cinnamomi-Paeonia lactiflora herb pair demonstrates potential efficacy in alleviating pain and depression17. Additionally, ginseng's total saponins can reduce hippocampal proinflammatory cytokine levels, improve depressive behavior, and attenuate hippocampal nerve damage in rats18. Licorice mainly contains triterpenoids and flavonoids. Licorice's total flavonoids (LF) can play an antidepressant role by improving depressive behavior, modulating the BDNF/TrkB signaling pathway, and enhancing synaptic plasticity19. However, the specific mechanisms underlying the antidepressant effects of QZF remain unclear, thereby limiting its widespread application.
Therefore, our study aims to establish a CRS depression rat model, demonstrate the therapeutic effect of QZF on depression in rats through behavioral experiments, and systematically evaluate the antidepressant mechanism of QZF using network pharmacology and molecular docking technology20. By clarifying the active components and potential targets of QZF, the core targets of depression can be accurately located. We believe that by deeply exploring the mechanism of action of QZF, we can not only provide safer and more effective treatment options for patients with depression but also provide a scientific basis for the application of TCM in the treatment of depression.
Protocol
All experimental protocols were approved by the Animal Experiment Ethics Committee of Shandong University of Traditional Chinese Medicine (approval number: YYLW2023000327) and complied with the Guide for the Care and Use of Laboratory Animals issued by the National Institutes of Health. In this experiment, we used 40 healthy male Wistar rats, SPF grade, with an average body weight of (140 ± 10) g (Figure 1). See the Table of Materials for a list of all the materials, equipment, and software used in this protocol.
1. Rat depression model
- Animal housing and grouping
- Enter the breeding room with the animals and number them using a tail marking instrument.
- House the rats individually in cages, maintaining a temperature of 21 ± 2 °C and a 12 h/12 h light/dark cycle.
- Acclimate the rats in the laboratory for 7 days, providing ad libitum access to food and water while handling them daily for adaptation.
- After the acclimation period, measure the body weight, and conduct the sucrose preference tests (SPT) and open field tests (OFT).
- Based on the experimental data, divide the rats into four groups, ensuring each group consists of 10 rats: the control (CON) group, the model (CRS) group, the QZF group, and the fluoxetine (F) group.
- Establishment of a Chronic Restraint Stress (CRS) rat model
- Construct the rat restraint device. Select a transparent plastic tube with a diameter and length suitable for the rat's size21, allowing the rat to stand and turn inside while preventing escape. Use an electric soldering iron hole puncher to create holes on the sides of the plastic tube and on the lid to ensure proper air circulation.
- Gently place the rats in the restraint devices (except for those in Group C) 1 h after daily intragastric administration of the drug, ensuring they are in a comfortable position.
- Deprive all groups of rats of food and water during the restraint period. After the restraint period ends, provide them with ample food and water uniformly. Fix the daily restraint duration at 6 h (from 9:30 h to 15:30 h) and maintain it for 28 consecutive days.
2. Drug intervention
- Administer via gavage: 1 mL of solution/100 g of body weight, fluoxetine (2.7 mg·kg-1·day-1) and QZF (2 g·kg-1·day-1)22. Provide Groups C and CRS with equivalent normal saline for single-variable control.
NOTE: Daily drug administration was conducted at 08:00 h, initiating synchronously with model establishment and persisting throughout the 28-day modeling period.
3. Sucrose preference test (SPT)
- Deprive the rats of food and water for 24 h before the experiment begins.
- Prepare a 1% aqueous sucrose solution and fill the solution and pure water into the drinking bottles of the experimental animals for weighing. Measure the consumption of pure water and sucrose water by weighing the bottles before and after the experiment.
- Place one bottle of sucrose solution and one bottle of pure water at the water intake of each rat cage cover, one on the left and one on the right, for free access to drinking. To prevent rats from favoring one side for water intake, switch the positions of the water bottles on the left and right after 30 min into the experiment.
- After 1 h of the experiment, remove all water bottles, weigh them promptly, and record the consumption of sucrose solution and pure water. Calculate the weekly sucrose preference ratio using the formula:
Sucrose preference value =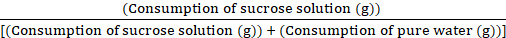 × 100%
× 100%
4. Body weight measurement
- Weigh the rats weekly upon their entry into the laboratory and set the fixed time for weighing at 7:00 AM.Establish this schedule to facilitate the observation of body weight changes.
5. Open-field test (OFT)
- Before the experiment starts, acclimate the rats to the behavioral room for 1 h and adjust the lighting in the open field box to ensure even distribution. Confirm that the rats are clearly visible in the tracking software.
- Utilize the video tracking and analysis system to divide the bottom surface of the open field box (50 cm x 50 cm x 50 cm) into nine equal-area square grids. Designate the eight grids adjacent to the walls as the peripheral area and the central grid as the central area.
- Place the rat in the central area of the open field box. Record the rat's movement for 5 min using the video tracking system.
- After testing each rat, clean the chamber with 75% ethanol to remove residual odor and prevent interference with the behavior of the next rat. Enter the total distance (mm) of open field activities and the number of entries in the central grid in the OFT records.
6. Forced swimming test (FST)
NOTE: The rat forced swimming experiment comprises a pre-experiment and a formal experiment. Conduct the pre-experiment 24 h prior to the formal experiment, following the same procedure, with the rat swimming for 15 min.
- Transport the experimental animals to the behavioral room at least 30 min before the experiment to allow them to acclimate to the environment.
- Prepare a transparent cylindrical plexiglass water cylinder (50 cm high, 20 cm diameter) and fill it with water at 23-25 °C. Adjust the water depth based on the animal's weight, ensuring the animal's tail remains a certain distance from the bottom of the cylinder.
- Slowly place the rats into the water cylinder and keep quiet throughout the experiment. Activate the camera and signal acquisition system. Observe and record the duration of floating immobility within 300 s. Immediately remove the rats from the water and dry them at the end of the experiment.
- After each session, replace the water to prevent any influence on the next rat.
7. Network pharmacological prediction
- Collection of QZF compounds and putative targets
- Access the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database (https://old.tcmsp-e.com/)23, the HERB database24, and the TCMID database (https://www.bidd.group/TCMID/). Use the eight TCM names in QZF, including GZ, YZ, YZR, BS, CBM, RS, HJT, and ZGC, as keywords to search for active compounds and targets of the herbs. Collect targets from the TCMSP and Swiss target prediction (http://www.swisstargetprediction.ch/). Set the filter value to Probability* > 0.
NOTE: Typically, ingredients were included as active ingredients based on their pharmacokinetic characteristics: oral bioavailability (OB) ≥ 30% and drug-like characteristics (DL) ≥ 0.1825.
- Access the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database (https://old.tcmsp-e.com/)23, the HERB database24, and the TCMID database (https://www.bidd.group/TCMID/). Use the eight TCM names in QZF, including GZ, YZ, YZR, BS, CBM, RS, HJT, and ZGC, as keywords to search for active compounds and targets of the herbs. Collect targets from the TCMSP and Swiss target prediction (http://www.swisstargetprediction.ch/). Set the filter value to Probability* > 0.
- Prediction of disease targets
- Search for the keyword "depression" in the GeneCards database (https://www.genecards.org/), obtain gene targets associated with depression, download the electronic spreadsheet of disease targets, filter the gene scores that are higher than the average value, and compile a list of depression targets26.
- Drug-component-disease-target network
- Create a new spreadsheet and populate it with depression-related targets and drug targets in the same column.Click on Start in the menu bar | Conditional Formatting | Highlight Cells Rules | Duplicate Values. Select a format (for example, "Light Red Fill") in the dialog box that appears27, click OK to view the results.
- Launch the network analysis software and import the spreadsheet file by clicking on File in the menu bar | Import | Network. Optimize the appearance of the network by adjusting the size and color of nodes in the Style panel located in the left control panel. Perform network topology analysis by clicking on Tools in the menu bar | Analyze Network27.
- Protein-protein interaction (PPI) network
- Access the Jvenn (https://jvenn.toulouse.inrae.fr/app/example.html) tool, upload the compound targets and disease targets separately, plot the Overlapping Genes (OGE) between the compound presumed targets and disease targets.Click on the numbers in the image and copy them into a spreadsheet and download the Venn diagram image.
- Access the STRING database (https://stringdb.org/)28, and enter the OGEs from the spreadsheet into the database. Specifically, paste the QZF anti-depression overlapping target list into the List of Names dialog box. Select Homo sapiens in the Organisms section and click on SEARCH | CONTINUE. Select the Exports option from the title bar and download the summary table of the PPI network in both PNG and TSV formats29.
- Screening of core proteins
- Start the network analysis software (https://cytoscape.org/). Then, in the menu bar, click on File | Import | Network | File to import the TSV format file generated in the previous step30.
- Select Analyze Network in the menu bar and click the Analyze button. Then, view the analysis results and understand the overall structural characteristics of the network, such as the number of nodes, the number of edges, and the average degree.
- Select Apps | App Manager in the menu bar. Search for MCODE, install the plug-in, and run the plug-in to get the hub target. Then, search for CytoNCA, install the plug-in, and focus on the three parameter values of Degree, Closeness centrality (CC), and Betweenness centrality (BC). According to the values of these parameters, screen Nodes with higher Degree, CC, and BC, which are generally considered core proteins31.
- Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis
- Open the bioinformatics platform (https://www.omicshare.com/). Click on the Tools menu, find the ID gene conversion tool, and click on it. Then, click the upload file button, select the generated step on the core of the target genes, and download the converted file ID list.
- In the Tools menu, click on the Dynamic KEGG enrichment Analysis tool. Upload the gene ID list. In the Species option, select Homo sapiens and click the Submit button.
- In the Tools menu, click on the Dynamic GO enrichment Analysis tool | Gene option | Upload file option. Select the gene ID list and in the Species option, select Homo sapiens. Select the GO type for analysis, including Biological Process, Molecular Function, and Cellular Component.
- For the results of KEGG and GO enrichment analysis, set the filtering threshold as p < 0.05. Arrange the counts in descending order.
8. Molecular docking verification
- Visit the PubChem (https://pubchem.ncbi.nlm.nih.gov/) website. Enter the target compounds in the search bar. Click on the 2D Structure and download it.
- Open the PDB (https://www.Rcsb.org/) database. Select the crystal structure with high resolution and containing the original ligand. Download the PDB file.
- For optimizing the protein structure, open the molecular visualization software. Load the downloaded PDB file. Remove water molecules and save the optimized PDB file.
- Open the molecular docking software and import the optimized PDB file. In AutoDockTools, click on Edit | Delete Water to delete water molecules. Click on Edit | Add Hydrogens | Add to add hydrogen atoms to the protein and ligand29.
- In AutoDockTools, set the receptor bar to receptor.pdbqt and the ligand bar to ligand.pdbqt. Open the pdbqt. file to view the binding sites of the protein and ligand and set the size and position of the docking box to ensure that it can completely enclose the receptor protein and the ligand compound. In AutoDockTools, click on Grid | Define Grid Box to set the center coordinates and dimensions of the box and use default values for molecular docking. The docking frames will be automatically sorted in descending order of binding energies.
- Open the result file and record the optimal binding energy value. Lower binding energies indicate more stable binding. Use the molecular visualization software to load the result file. Adjust the view and color to clearly display the ligand-receptor interaction.
9. Statistical analysis
- Conduct statistical analysis in the scientific data analysis and visualization software and represent all data as the mean ± SEM. Use repeated measures two-way ANOVA for comparisons between groups before and after drug administration. Employ one-way ANOVA for comparisons among more than two groups.
- Take the value of P < 0.05 as statistically significant.
Results
Behavioral test results in the CRS-induced rat depression model
Sucrose preference test results
At baseline, there was no difference in the sucrose preference coefficient among the groups (P > 0.05). Following 28 days of intervention, the sucrose preference coefficient of the CRS group was significantly lower than that of the CON group (P < 0.05), while the F and QZF groups showed significantly higher coefficients compared to the CRS group (both P < 0.01).The results indicated that stressed rats exhibited typical anhedonic symptoms, which were alleviated by treatment with F and QZF (Figure 2A).
Body weight results
Before CRS induction, no significant differences were observed among the groups (P > 0.05).After 4 weeks of stress, the body weight growth rate of group CRS was significantly lower than that of group CON (P < 0.01), while groups F and QZF exhibited significantly higher growth rates than group M (P < 0.001, P < 0.01).These findings indicate that stress disrupted the normal physiological metabolism in rats, with the F and QZF groups showing significant improvements and corrections in their abnormal metabolic profiles (Figure 2B).
Open field test results
After 28 days of intervention, there was no significant difference in the total distance of open field test among the four groups (P > 0.05) (Figure 2D). Compared with group CON, the time spent in the central area of group CRS was significantly reduced (P < 0.01). Compared with the CRS group, the time spent in the central area of the F and QZF groups was significantly increased (both P < 0.01). There was no significant difference between the treatment groups (P > 0.05) (Figure 2C,E).
Forced swimming test results
After 28 days of intervention, the CRS group exhibited a significantly increased immobility time compared to the CON group (P < 0.0001).Compared to the CRS group, the F and QZF groups showed significantly reduced immobility times (P < 0.05, P < 0.001) (Figure 2F).
Network pharmacology prediction
Target networks with composite assumptions
To construct the QZF-compound-hypothetical target network, we first screened 1,020 hypothetical targets of QZF, which were collected and visualized as compound targets by the network analysis software. The network showed 1,184 nodes and 8,728 edges (Figure 3)32.
QZF and depression target screening
A total of 17,947 depression-related targets were retrieved from the GeneCards database, exhibiting an average relevance score of 1.105. Targets with a Relevance Score exceeding 1.105 (n = 5,048) were subsequently selected for further data analysis. A Venn diagram was constructed with 1,020 targets from QZF to obtain 612 common targets (OGEs) (Figure 4A). The 612 common targets were imported into the STRING database for analysis, and the PPI network contained 607 nodes and 14,375 edges (Figure 4B), and the OGEs were imported into the network analysis software to obtain the interaction network.
Screening of core target genes
Module analysis using the MCODE plugin identified the cluster module with the highest score, which had an MCODE score of 54.1902931. We identified 64 key targets within the cluster hub module that are critical for QZF's antidepressant effects (Figure 4C). Using the CytoNCA plugin, we screened for highly connected nodes based on three centrality metrics: Degree Centrality (DC), Closeness Centrality (CC), and Betweenness Centrality (BC). Specifically, degree centrality measures the number of direct connections a node has within the network. Closeness centrality quantifies the reciprocal of the average shortest path length between a node and all other nodes, indicating how efficiently a node can access others. Betweenness centrality evaluates the frequency with which a node appears in the shortest paths between all pairs of nodes, reflecting its mediating role. Based on these metrics, we constructed the core network and identified the top 10 most connected nodes: BCL2, AKT1, IL6, BCL2L1, MTOR, CASP3, TP53, STAT3, NFKB1, and HIF1A (Figure 4D). Following data filtering, we performed functional enrichment analysis on these 10 key target genes to further elucidate their biological functions.
GO enrichment analysis
The GO enrichment analysis yielded a total of 2,783 annotated items, with 2,385 exhibiting statistical significance. This analysis predominantly influenced the biological process (BP), molecular function (MF), and cellular component (CC) categories. Specifically, the GO-BP category encompassed 2,450 items, of which 1,926 were deemed statistically significant. The molecular function (GO-MF) category identified 184 items, with 117 showing statistical significance. The cellular component (GO-CC) category revealed 149 items, and among these, 59 were statistically significant (Figure 5).
KEGG enrichment analysis
The KEGG pathway enrichment analysis identified a total of 156 pathways associated with the 10 key targets, with 119 of these pathways demonstrating statistical significance. The figures illustrate the top 20 pathways with the highest enrichment scores (Figure 6). Removal of some associated diseases left two signaling pathways, HIF-1 and JAK-STAT signaling pathways, which were predicted to be key pathways for QZF and depression.
Major target-pathway networks for QZF and Depression
To elucidate the mechanistic relationship between QZF and its effects on depression, we developed a pivotal TCM-compound-target-pathway interaction network (Figure 7). Utilizing network analysis software, we visualized the signaling pathway with the most significant p-value along with its associated targets. The resulting network graph comprised 93 nodes and 218 edges. Furthermore, we generated a Sankey diagram to represent key genes and their corresponding active compounds, specifically focusing on the HIF-1 and JAK-STAT core signaling pathways (Figure 8).
Molecular docking
Molecular docking analysis was adopted to substantiate the target specificity of the compound. This technique assesses the binding affinity between a ligand and its protein target, where lower magnitudes of binding energy indicate a stronger interaction and closer proximity of the ligand to its binding site33. The outcomes revealed that the binding energies were -8.7 kcal/mol for HIF1A and Glycyrrhiza flavonol A, -8.5 kcal/mol for STAT3 and Ginsenoside rh2, -7.6 Kcal/mmol for BCL2 and Isolicoflavonol, -6.8 Kcal/mol for MTOR and Licochalcone B, -6.7 Kcal/mol for AKT1 and Kaempferol, and -5.2 Kcal/mol for IL6 and Linolenic acid.
Overall, the molecular docking results demonstrated that the compounds exhibited a strong binding affinity for their targets. The binding energy of each protein is visualized as follows: the white cartoon pattern represents the protein receptor, the blue one is the small molecule ligand, the yellow dotted line indicates the hydrogen bond formed between the ligand and the receptor, the green represents the attachment site of the hydrogen bond between the protein receptor and the small molecule ligand, and the numbers signify the hydrogen bonding distances, which implies that the binding between the ligand and the receptor is highly stable (Figure 9)34.
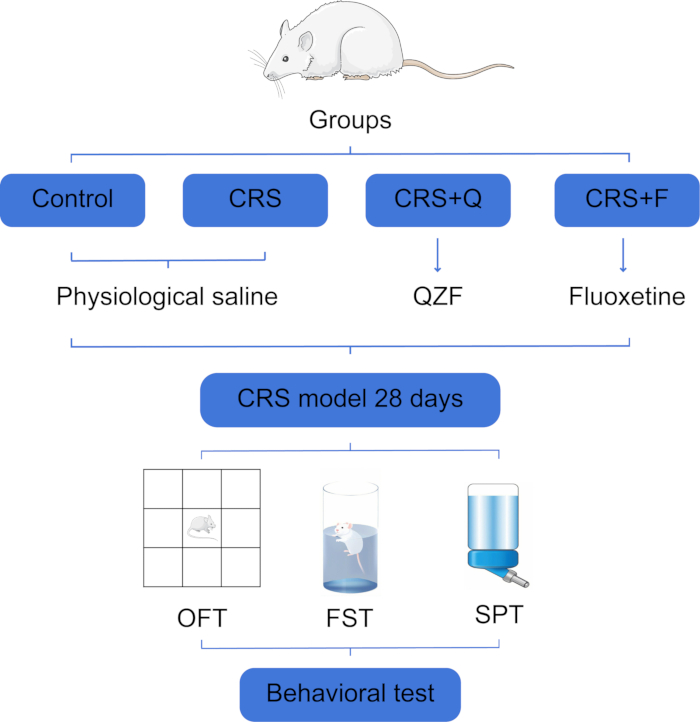
Figure 1: Flowchart of grouping and behavioral tests for experimental rats. Abbreviations: CRS = chronic restraint stress; QZF (Q) = qiangzhifang; F = fluoxetine; OFT = open field test; FST = forced swimming test; SPT = sucrose preference test. Please click here to view a larger version of this figure.

Figure 2: Effects of QZF on the CRS-induced rat depression model. (A) Sucrose consumption level (%) on day 0 and day 28. (B) Body weight (g) on day 0 and day 28. (C) Plot of rat trajectories in the open field test at week 4. (D) Open field total distance on days 0 and 28. (E) The duration of staying in the central area of OFT in each group at week 4. ** P < 0.01 indicates a significant difference between the F and QZF groups compared to the CRS group. (F) The FST immobility time (%) in each group at week 4. * P < 0.01 indicates a significant difference between the F group compared to the CRS group. *** P < 0.001 indicates that the QZF group showed significant differences compared with the CRS group. Abbreviations: CRS = chronic restraint stress; QZF = qiangzhifang. Please click here to view a larger version of this figure.
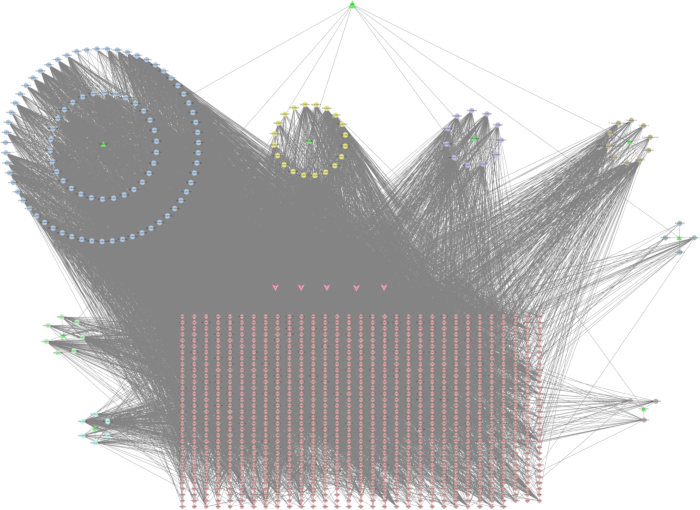
Figure 3: QZF-Compound-Target network. The green triangles denote the traditional Chinese medicines in QZF; the circles denote the components of the traditional Chinese medicines; the rhombuses denote the targets. The pink arrows indicate the common constituents of several Chinese herbal medicines. A (MOL000211) relates to Bai shao and Zhi gan cao; B (MOL000358) is associated with Bai shao, Chuan bei mu, Gu zhi, and Ren shen; C (MOL000359) connects with Bai shao, Chuan bei mu, and Gui zhi; D (MOL000422) pertains to Bai shao, Zhi gan cao, and Ren shen; E (MOL000492) is relevant to Bai shao and Gu zhi. Please click here to view a larger version of this figure.
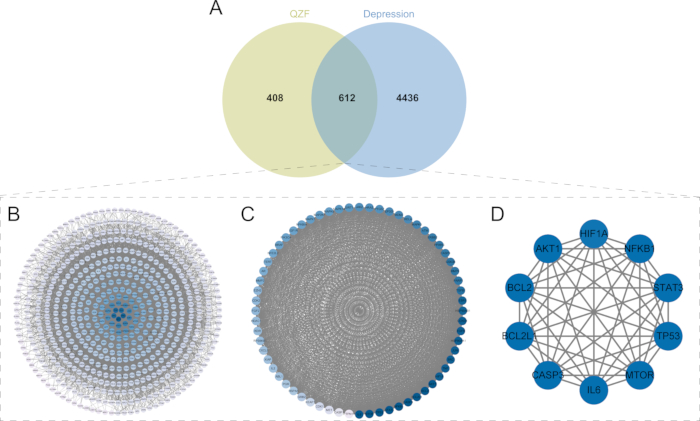
Figure 4: Identification of intersection targets and screening of the core targets. (A) Venn diagram of the common targets of QZF and depression.Light green circles represent the target proteins of the active ingredients in QZF; blue circles denote proteins associated with depression. The overlapping areas, where the two colors intersect, illustrate the shared proteins, totaling 612. (B) PPI network of QZF and depression. (C) MCODE analysis. (D) Top 10 core targets. Abbreviations: QZF = qiangzhifang; PPI = protein-protein interaction. Please click here to view a larger version of this figure.

Figure 5: Histogram for the GO enrichment analysis of common targets. The green bars represent biological processes; the red bars represent molecular functions; the blue bars represent cellular components. The height of each bar reflects the gene count associated with the corresponding GO term. Abbreviation: GO = Gene Ontology. Please click here to view a larger version of this figure.

Figure 6: KEGG enrichment pathways of QZF's therapeutic targets in depression. (A) Bar chart of the top 20 pathways, ranked by P-value. (B) Bubble chart of the top 20 pathways: point size indicates gene number; color intensity reflects P-value significance. (C) Functional annotation of KEGG pathways. Abbreviation: KEGG = Kyoto Encyclopedia of Genes and Genomes. Please click here to view a larger version of this figure.
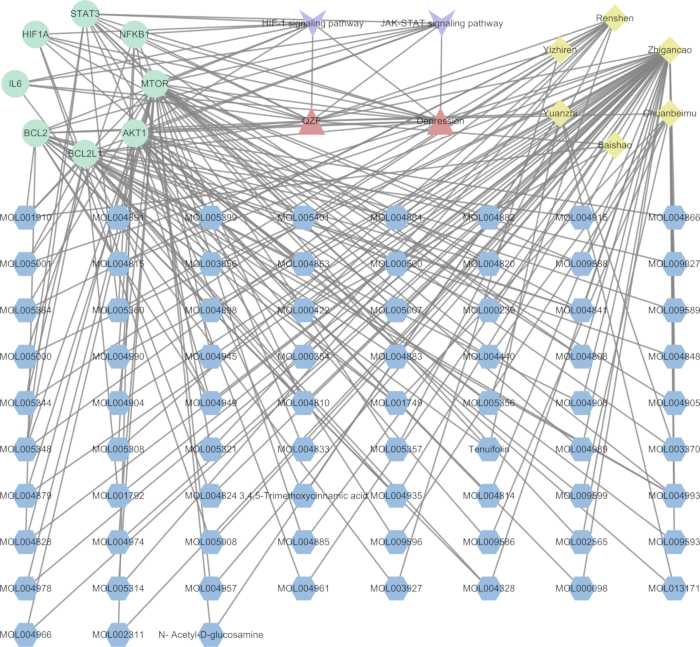
Figure 7: Interaction network of TCM-compound-target-pathway. Red denotes QZF and depression, purple designates signaling pathways, green highlights core pathway proteins, yellow identifies traditional Chinese medicines within QZF, and blue specifies constituent herbal compounds. Please click here to view a larger version of this figure.
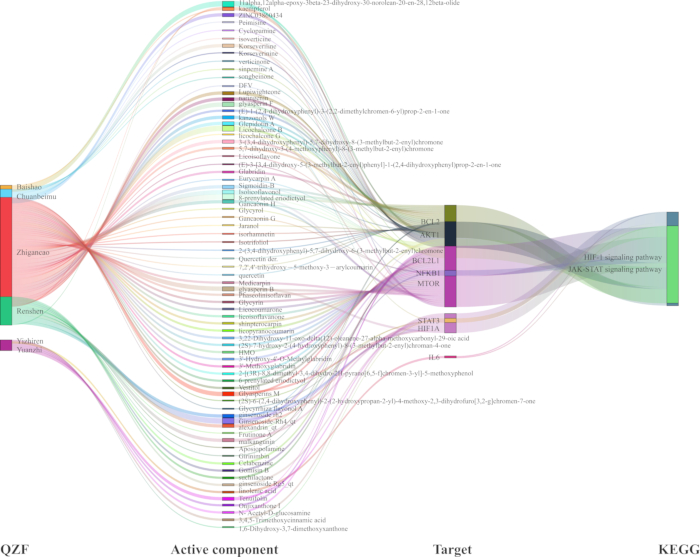
Figure 8: Sankey diagram of the TCM-compound-target-pathway for the antidepressant effect of QZF based on HIF-1 and JAK-STAT signaling pathways. Abbreviations: QZF = qiangzhifang; TCM = Traditional Chinese medicine; HIF-1 = hypoxia-inducible factor-1; JAK-STAT = Janus-activated kinase-signal tranducers and activators of transcription. Please click here to view a larger version of this figure.
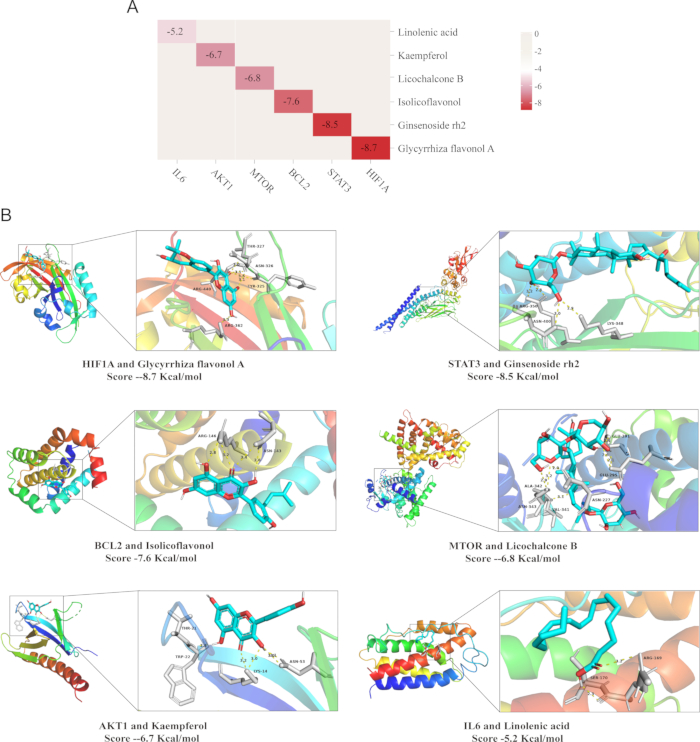
Figure 9: Molecular docking validation results. (A) Heat map of binding energy (kcal/mol) between representative components of QZF and target protein molecules (B) Visualization of docking situation. Abbreviation: QZF = qiangzhifang. Please click here to view a larger version of this figure.
Supplemental File 1: Preparation of QZF traditional Chinese medicine granules. Abbreviation: QZF = qiangzhifang. Please click here to download this File.
Discussion
CRS is a widely used method for establishing animal models of depression. This model mimics the chronic psychological stress encountered in human life and induces depression-like behaviors in rats35. In this study, the rat restraint tube was constructed from transparent plastic, ensuring animal safety while enabling clear observation during the experiment. The transparent tube measured approximately 18 cm in length and 6 cm in diameter and featured multiple ventilation holes, each with a diameter of 1 cm, uniformly distributed along the sides and lid to provide sufficient airflow for the rats. The stressed rats exhibited depressive symptoms such as lethargy and glassy eyes, along with behavioral changes characteristic of depression. Specifically, these changes included decreased motor activity in the OFT, prolonged immobility time in the FST, and reduced sucrose consumption in the SPT. These behavioral manifestations closely resemble the bradykinesia, anhedonia, and loss of interest observed in patients with clinical depression.
In the context of studying the complex pathological mechanisms of depression, the combination of network pharmacology and molecular docking technology provides an innovative strategy for analyzing the molecular mechanisms of traditional Chinese medicine compounds in the treatment of depression.This study identified HIF1A, STAT3, BCL2, MTOR, AKT1, and IL6 as the core targets of QZF in the treatment of depression.These targets were primarily enriched in the HIF-1 and JAK-STAT signaling pathways.These two signaling pathways play a central role in the key pathological processes of depression, such as neuroinflammation, oxidative stress, and apoptosis.
The HIF-1 signaling pathway, serving as the central regulatory mechanism for cellular oxygen metabolism, plays a crucial role in various physiological processes, including neuroprotection, antioxidant stress responses, and angiogenesis36. Research indicates that the brain tissue of individuals with depression exhibits a pronounced hypoxic microenvironment and oxidative stress injury, which are closely associated with the activation of neuroinflammatory responses and the imbalance of neurotransmitters37. Semenza's research demonstrates that under hypoxic conditions, HIF-1α upregulates genes associated with oxygen metabolism and antioxidant defense mechanisms, including vascular endothelial growth factor (VEGF), erythropoietin (EPO), and mitochondrial genes. Consequently, this enhances mitochondrial function, promotes the formation of brain microvessels, increases oxygen delivery to brain tissue, and reduces reactive oxygen species (ROS) accumulation38.
Further experimental studies demonstrate that HIF-1α deficiency markedly enhances neuronal susceptibility to oxidative stress, thereby triggering abnormal activation of the apoptotic signaling pathway39. This leads to a significant increase in neuronal apoptosis and progressive cognitive decline. In contrast, neuron-specific HIF-1α overexpression in transgenic mouse models significantly boosts both neuronal survival and synaptic density40. These findings not only substantiate the critical role of HIF-1α in the antioxidant defense mechanism but also highlight its potential therapeutic significance in enhancing brain function through the promotion of neural plasticity remodeling and optimization of synaptic architecture. Moreover, the HIF-1 signaling pathway antagonizes the NF-κB signal transduction pathway, leading to a reduction in the production of inflammatory cytokines IL-6 and TNF-α, suppression of neuroinflammation, and the exhibition of potential neuroprotective and antidepressant effects41.
Notably, Glycyrrhiza flavonol A, one of the active components of QZF, has been confirmed to exhibit antioxidant and anti-inflammatory properties. In this study, molecular docking data reveal that liquiritigenin A exhibits a high binding affinity to HIF-1α protein, reaching -8.7 kcal/mol. This finding strongly indicates that Glycyrrhiza flavonol A, may directly target HIF-1α, modulating its protein stability or transcriptional activity. Consequently, it regulates the expression of genes involved in oxygen metabolism and antioxidant defense within the HIF-1 signaling pathway, thereby enhancing neuronal survival under hypoxic conditions and alleviating depression-associated neural damage.
The JAK-STAT signaling pathway serves as the central hub for cytokine signal transduction and plays a pivotal role in various biological processes, including inflammation regulation, immune response modulation, and neuronal survival42,43. Extensive research has demonstrated that the pathogenesis of depression is intricately linked to the dysregulation of the JAK-STAT signaling pathway44. A meta-analysis performed by Dowlati et al. revealed that, compared with healthy controls, serum levels of pro-inflammatory cytokines such as IL-6 and TNF-α were significantly increased in patients with depression and positively correlated with the severity of depressive symptoms45. Specifically, these pro-inflammatory factors are capable of activating the JAK-STAT pathway, thereby eliciting an inflammatory response. This process not only induces direct damage to neurons and glial cells but also compromises synaptic structure and function, ultimately exacerbating cognitive and emotional impairments in patients46.
Moreover, the excessive activation of the JAK-STAT pathway is strongly associated with neuronal apoptosis. Sustained STAT3 phosphorylation upregulates the expression of pro-apoptotic genes, including members of the Caspase family, ultimately resulting in neuronal loss. Additionally, the aberrant activation of this pathway impairs neurogenesis in the hippocampal region and diminishes synaptic plasticity, thereby exacerbating neurofunctional deficits47. In this study, ginsenoside Rh2, an important active component of QZF, exhibited significant binding affinity with STAT3 protein in the molecular docking analysis. Based on these findings, ginsenoside Rh2 may effectively alleviate neuroinflammatory responses by specifically inhibiting STAT3 activation and thereby reducing the production and release of pro-inflammatory cytokines48.
In addition to the two core signaling pathways, HIF-1 and JAK-STAT, this study identified the synergistic interactions among other active components and targets during the antidepressant action of QZF. BCL2, a canonical anti-apoptotic protein, plays an essential role in sustaining cell survival and suppressing apoptotic signaling pathways49. In QZF, isolicoflavonol exhibits antioxidant and anti-apoptotic properties by specifically targeting and activating the BCL2 protein, thereby effectively inhibiting neuronal apoptosis, protecting neurons, and ameliorating neuro-pathological alterations associated with depression. Furthermore, the aberrant MTOR signaling pathway in patients with depression is strongly associated with neuronal dysfunction50. Studies have demonstrated that Licochalcone B promotes neuronal growth and survival, enhances synaptic plasticity and functional connectivity by modulating the MTOR signaling pathway51, thereby exerting an antidepressant effect. In addition, the natural flavonoid kaempferol, characterized by its potent antioxidant and anti-inflammatory activities, specifically activates the AKT1 signaling pathway. Through the regulation of multiple key downstream molecules, it not only promotes neuronal survival but also accelerates functional recovery, thereby providing additional molecular target support for the antidepressant effects of QZF.
In summary, this study utilized network pharmacology and molecular docking to predict the therapeutic pathways, core targets, and effective active components of QZF in treating depression. The antidepressant effect of QZF was validated in a rat model of depression, suggesting that it may exert its antidepressant effects by modulating multiple signaling pathways, including HIF-1 and JAK-STAT, and targeting key pathological processes such as neuroinflammation, oxidative stress, and apoptosis. This finding not only deepens our understanding of the pathological mechanisms underlying depression but also provides a theoretical foundation and novel therapeutic targets for the application of traditional Chinese medicine formulas in depression treatment. However, this study has certain limitations. The synergistic mechanisms of multiple components in QZF remain to be fully elucidated, and the metabolic processes and interactions of these components in vivo require further investigation. Future research could integrate in vitro and in vivo experiments with advanced technologies such as liquid chromatography, high-throughput sequencing, and multi-omics integration to comprehensively and accurately identify the key targets and pathways associated with the antidepressant effects of QZF, thereby verifying and expanding the predictions made through network pharmacology.
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgements
The research was supported by the National Natural Science Foundation of China (82374311), the State Administration of Traditional Chinese Medicine High-Level Traditional Chinese Medicine (TCM) Basic Theory Key Discipline Construction Project (zyyzdxk-2023118), the National Traditional Chinese Medicine Experts Studio Construction Project (National Chinese Medicine Education Letter No.75) and the Natural Science Foundation of Shandong Province (ZR2022LZY016). QZF granules were prepared by the Department of Pharmaceutical Products, Affiliated Hospital of Shandong University of Traditional Chinese Medicine.
Materials
| Name | Company | Catalog Number | Comments |
| Animal behavior analysis system | Shanghai Xinsoft Information Technology Co., LTD | XR-SuperMaze | |
| AutoDockTools | The Scripps Research Institute | ||
| Cytoscape software | Cytoscape Consortium | version 3.7.2 | |
| Electric soldering iron hole puncher | Nanjing Naiwei Technology Co., Ltd. | ||
| Fluoxetine | Lilly Suzhou Pharmaceutical Co., LTD | ||
| Open field experimental system | Shanghai Xinsoft Information Technology Co., LTD | XR-XZ301 | |
| PyMol | Schrödinger | ||
| Qiangzhifang | Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, China | ||
| Transparent plastic tube | Nantong Baiyang Plastic Products Co., Ltd. |
References
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved