需要订阅 JoVE 才能查看此. 登录或开始免费试用。
Method Article
用于光催化活性的金属氧化物异质结
摘要
异质结的发展提高了溶液燃烧合成的光催化活性,这是一个省时/节能的过程。该方案中使用了先进的分析表征技术来评估材料的特性,纳米复合材料显示出酸性橙-8 染料降解的改善。
摘要
全球对合成技术及其最佳特性的改进有巨大的需求,尤其是对于工业规模的应用。基于溶胶-凝胶的溶液燃烧合成 (SG-SCS) 是一种生产有序多孔材料的简单方法。在这方面,Pearson 的硬酸和软酸碱理论有助于选择宿主-掺杂剂反应性以形成适当的异质结。
异质结的形成还改变了材料的基本性质,通过电荷转移或协同活性改善了光催化。根据通过差重比分析 (DTG) 进行的稳定性评估结果,500 °C 的煅烧温度是此过程的理想选择。
使用 X 射线衍射和高分辨率透射电子显微镜 (HRTEM) 验证生成的纳米颗粒 (NPs) 和纳米复合材料 (NCs) 的纳米级尺寸。此外,扫描电子显微镜显微照片和 BET 分析证实了材料的孔隙率性质。高分辨透射电镜、X 射线光电子能谱和能量色散 X 射线研究确定了材料的组成。研究发现,NC 比裸 ZnO 更有效地降解酸性橙 8 (AO8) 颜色。
引言
随着全球公司的迅速崛起,环境保护已成为一个主要问题。因此,基于纳米技术的纳米材料 (NMs) 及其合成在现代科学界引起了研究人员对块状材料的关注1。几种物理化学方法已被用于处理有机和无机污染物 2,3。在这方面,由于其简单性和溶解毒素而不产生二次污染的能力,非均相光催化被认为是一种适应性修复技术4。研究已经在合适的带隙半导体之间设计了异质结或掺杂,这有助于减少成分的电子-空穴复合、表面积和体积。这种情况随后增加了染料的光催化降解 5,6,7。最近的工作还报道了通过异质结/混合产生协同作用和充电器转移改进作用 8,9,半导体金属氧化物在多功能应用中表现出独特的物理和化学特性10。因此,TiO2 和氧化锌 NPs (ZnO NPs) 在研究人员中受到了极大的关注11,12。
与单一材料相比,异质结的形成已成为增加材料表面积和体积比以及提高材料光催化和抗菌性能的独特偏好之一。此外,与二元异质结相比,二元异质结的协同作用改善了光生电子/空穴对的分离 13,14。研究表明,Mn2O3 和 ZnO NPs15 之间的异质结提高了合成 NPs 的稳定性和底物吸附能力,并降低了电荷转移电阻。此外,一些研究使用基于 Pearson 的硬质和软质酸碱 (HSAB) 理论的宿主-掺杂剂反应性来测试异质结或掺杂剂的形成。研究发现,在水等硬碱溶剂存在下,硬路易斯酸(如 Mn(III))不能扩散到 Zn (II) 主体晶格的边界16,17。它们被吸附到主体表面并在煅烧时被氧化形成杂化物。
由于其潜力,目前全球材料合成工业可扩展应用的重点是改进该方法及其关键前景13。固溶燃烧合成 (SCS) 是一种简单的、省时省能的方法,可以产生规则有序的多孔材料18,这些材料在离子/质量传递现象中起着重要作用19。SCS 包括基于 Pearson 的硬酸和软酸碱 (HSAB) 理论的不错的掺杂剂-宿主分布或异质结。掺杂/异质结可以调整材料的光学、磁性和电学特性,随后通过有效的电荷转移和/或协同作用促进材料的应用20。结构导向剂 (ADA) 辅助的 SCS 还可以生产有序的胶体纳米晶体框架 (CNF),用于能量转换器件中的质量/离子传输21,22。
本研究通过环保的 SG-SCS 方法制备了一种聚乙烯醇 (PVA) 表面活性剂和络合剂,用于合成 ZnO NPs 和基于 ZnO 的二元纳米复合材料 (NCs) 异质结。氧化物之间的异质结在电荷转移中起着至关重要的作用,是根据 HSAB 理论估计的。利用表征技术来了解材料的结构、光学和形态特性。该材料的降解效率在稳定和有毒的 AO8 染料上进行了测试。
研究方案
1. 纳米材料合成
- ZnO-Mn2O3 纳米复合材料合成
- 使用聚乙烯醇作为表面活性剂和络合剂辅助 SG-SCS 方法合成纳米复合材料。有关 SG-SCS 方法的图形说明,请参见 补充图 S1。
- 将 1.5 g PVA 聚合物溶解在 100 mL 蒸馏水中,并在磁力搅拌器上在 115 °C 下持续搅拌约 15 分钟23。
- 将盐前驱体溶液、浓度为 90% v/v 的硝酸锌六水合物和浓度为 10%v/v 的硫酸锰倒入上述溶解的 PVA 溶液中,连续搅拌约 10 分钟,并将温度降至 70 °C。
注:盐前驱体同时混合以平衡纳米复合前驱体的反应性,以遵循成核-掺杂方法16,24。按照 La Mar 模型25,26,将温度降低到 70 °C 以控制纳米颗粒的加速生长和聚集。 - 将金属氢氧化物的显出溶胶(胶体颗粒)置于封闭和黑暗区域 2 天,使其老化。然后,通过在 110 °C(空气中)加热以形成凝胶来脱水溶液。
注:PVA 聚合物充当结构,引导模板和络合剂,有助于金属阳离子的均匀分散,启动燃烧过程并防止聚集/团聚特性。 - 通过将烘箱加热至 ~150-250 °C 的点火温度(使用简单温度计检查近似温度),使凝胶在空气中燃烧。点火温度是开始燃烧所需的最低温度。燃烧时,使用罩收集所有影响人体健康的有毒气体副产品。
注:通过在 PVA 聚合物和硝酸盐前体之间形成复合物来激活燃烧过程,这些复合物充当燃料以促进燃烧过程。 - 在马弗炉中将燃烧的材料在 500 °C 下煅烧 3 小时,使用差重 (DTG) 分析技术进行优化。DTG 分解未燃烧的杂质并提高材料的结晶度27.
- 裸 ZnO 和 Mn2O3 NPs 合成
- 使用溶胶-凝胶方法合成裸金属氧化物。使用前面提到的所有步骤,步骤 1.1.1.-1.1.6.,除了步骤 1.1.2 之外,在没有 PVA 的情况下合成裸露的 ZnO 和 Mn2O3 。由于不存在金属硝酸盐和 PVA 聚合物复合物,因此在最后的干燥步骤中不会发生自传播过程。
2. NP 表征
- 在氮气气氛中以 20.0 mL/min 的流速和 50 °C/min 的升温时间确定热重比率,特别是热热重/差热(DT/DTA 分析),以研究 NP 和 NC 的热稳定性和降解行为。
- 使用 400-4000 cm-1 范围内的 KBr 颗粒进行傅里叶变换-红外光谱 (FTIR),以研究 NPs 和 NCs 的表面官能团行为。
- 进行 X 射线衍射 (XRD) 以研究 PVA、NP 和 NC 的晶体结构。
- 使用 Brunauer-Emmett-Teller (BET;N2 吸附-脱附等温线)方法计算样品在相对压力 (P/Po) 范围内 0.05-0.35 的比表面积。使用 Barrett-Joyner-Halenda (BJH) 方法确定样品的孔径分布。最后,在 −196.15 °C 下测量所有 NPs 和 NCs 的 N2 吸附。
- 使用扫描电子显微镜与能量色散 X 射线光谱 (SEM-EDX) 和高分辨率透射电子显微镜 (HRTEM) 来研究 NPs 和 NCs 的形态并进行成分研究。
- 在集成了 Kratos 专利磁浸透镜、电荷中和系统和球面镜分析仪的系统上执行 X 射线光电子能谱 (XPS) 分析。根据外部碳的能量校准峰值能量。
注:研究人员在表征过程中采用了所有标准程序和协议。
3. 批量降解研究
- 通过将 20 ppm 的 AO8 染料溶解在 250 mL 的水溶液(水溶剂)中,用 0.06 g ZnO NPs 和 NCs 光催化剂进行光催化实验。
- 将降解实验用作 176.6 cm2 圆形玻璃反应器中的导体。在本实验中,使用中压汞蒸气灯(Hg 灯)作为光源(λmax = 365 nm,125 W)28。照明前,在黑暗中连续搅拌反应悬浮液 30 分钟,以在 NPs/NCs 上产生 AO8/CR 的吸附/解吸平衡。
- 通过将光从 20 cm 的距离聚焦在反应混合物上,直接照射样品。使用 110 rpm 的磁力搅拌器连续混合溶液。在实验过程中,利用水循环控制整个反应器的温度。
- 每 15 分钟吸取 5 mL 染料溶液,以通过 UV-vis 分光光度计在时间 t 测量其浓度。使用以下公式计算光催化降解效率的百分比:
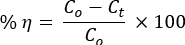
其中 Co 和 Ct 分别是 AO8 和 CR 染料溶液的初始和时间后 t 照射浓度;η是光脱色效率, - 使用准一阶动力学方程研究反应动力学:
伪 - 一级动力学: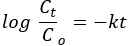
其中 Co 和 Ct 分别是 AO8 染料的初始浓度和平衡浓度 (mg/L),k 是速率常数, t 是以分钟为单位的时间。
结果
图 1A 描述了 DTG 仪器分析 N2 气氛中煅烧之前二元 NC 的热稳定性。吸附的 H2O 分子汽化、分子内衰变、金属氢氧化物或/和 PVA 侧链分解、分子间/PVA 主链分解,最后发生结晶部分,得到碳、碳氢化合物和灰分29,30。
NCs 在 720 °C 以上表现出稳定性损失。 XRD 图谱的衍射角?...
讨论
本方案描述了使用具有精确形状、尺寸和结构的自下而上的策略合成纳米晶体。该研究观察到,纳米晶体的成核和生长在形成纳米晶体之前是显着的。在这里,ZnO 和锰氧化物是基于 LaMer 的群论25 合成的,该理论假设纳米晶体在将前驱体还原成原子和原子核后形成纳米晶体,导致种子形成以产生纳米晶体。在这方面,纳米晶体的整体形状和大小取决于种?...
披露声明
作者没有什么可披露的。
致谢
我们要感谢安道麦科技大学对这项工作的支持。资金由沙特阿拉伯塔伊夫泰夫大学泰夫大学研究人员支持项目编号 (TURSP-2020/44) 提供。
材料
| Name | Company | Catalog Number | Comments |
| Acid orange 8 | Sigma-Aldrich | 65%, | |
| Chlorine | Sigma-Aldrich | 7782-50-5 | |
| Dithienogermole | Sigma-Aldrich | 773881-43-9 | |
| HCl | Sigma-Aldrich | 7647-01-0 | |
| Manganese nitrate (10%) salt | Sigma-Aldrich | 15710-66-4 | 10% |
| Manganese sulfate monohydrate | Sigma-Aldrich | Density: 2.95 g/cm³; solubility in water: 70 g/100 mL (70 °C); 99.95%, MnSO4.H2O | |
| Poly (vinyl alcohol) | Sigma-Aldrich | 9002-89-5 | Density: 1.19–1.31 g/cm³ @20 °C, soluble in water only @ > 80 °C |
| Zinc nitrate hexahydrate (90%) | Sigma-Aldrich | 10196-18-6 | 98%; Density: 2.065 g/cm³ @20 °C; solubility in water: 184.3 g/100 mL @20 °C |
| Instruments used | |||
| Materials name | Model | Analysis | |
| BET (N2 adsorption-desorption isotherms) | Quanta chrome instrument. | Textural properties | |
| DT/DTA | Shimadzu DTG-60H | Measure thermal stability | |
| FTIR | Perkin Elmer FT-IR, Spectrum 65 | Chemical bonding information | |
| HRTEM | JEOL TEM 2100 HRTEM | Morphological, size, and composition analysis | |
| SEM-EDX | SEM-EDX-EVO 18 with low vacuum facility and ALTO 1000 cryo attachment | Morphological analysis | |
| XPS | AXIS ULTRA from AXIS 165 | ||
| XRD | Shimadzu, XRD-7000 | Crystallinity, structure, and approximate average crystallite size | |
| Common software used | |||
| Name | Company | Use | |
| Mendeley | Mendeley-Desktop-1.19.8-win32 | For citing references | |
| Origin | OriginPro 8 | XRD, BET, UV-vis-DRS data analysis |
参考文献
- Khort, A., et al. Corrosion and transformation of solution combustion synthesized Co, Ni and CoNi nanoparticles in synthetic freshwater with and without natural organic matter. Scientific Reports. 11 (1), 7860 (2021).
- Pype, M., Lawrence, M. G., Keller, J., Gernjak, W. Reverse osmosis integrity monitoring in water reuse: The challenge to verify virus removal - A review. Water Research. 98, 384-395 (2016).
- Adeleye, A. S., et al. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chemical Engineering Journal. 286, 640-662 (2016).
- Gómez-Pastora, J., et al. Review and perspectives on the use of magnetic nanophotocatalysts (MNPCs) in water treatment. Chemical Engineering Journal. 310 (2), 407-427 (2017).
- Nadeem, M. S., et al. Enhancement in the photocatalytic and antimicrobial properties of ZnO nanoparticles by structural variations and energy bandgap tuning through Fe and Co co-doping. Ceramics International. 47 (8), 11109-11121 (2021).
- Nadeem, M. S., et al. Energy-levels well-matched direct Z-scheme ZnNiNdO/CdS heterojunction for elimination of diverse pollutants from wastewater and microbial disinfection. Environmental Science and Pollution Research International. , (2022).
- Munawar, T., Iqbal, F., Yasmeen, S., Mahmood, K., Hussain, A. Multi metal oxide NiO-CdO-ZnO nanocomposite-Synthesis, structural, optical, electrical properties and enhanced sunlight driven photocatalytic activity. Ceramics International. 46 (2), 2421-2437 (2020).
- Srinivasa, N., et al. Facile synthesis of Ni/NiO nanocomposites: The effect of Ni content in NiO upon the oxygen evolution reaction within alkaline media. RSC Advances. 11 (24), 14654-14664 (2021).
- Chen, P., et al. Solution combustion synthesis of ternary Ni/WC/C composites with efficient electrocatalytic oxygen reduction performance. RSC Advances. 11 (61), 38718-38726 (2021).
- Nagvenkar, A. P., Perelshtein, I., Piunno, Y., Mantecca, P., Gedanken, A. Sonochemical one-step synthesis of polymer-capped metal oxide nanocolloids: Antibacterial activity and cytotoxicity. ACS Omega. 4 (9), 13631-13639 (2019).
- Janotti, A., Van de Walle, C. G. Fundamentals of zinc oxide as a semiconductor. Reports on Progress in Physics. 72 (12), 126501 (2009).
- Abebe, B., Murthy, H. C. A., Amare, E. Enhancing the photocatalytic efficiency of ZnO: Defects, heterojunction, and optimization. Environmental Nanotechnology, Monitoring. & Management. 14, 100336 (2020).
- Abebe, B., Murthy, H. C. A., Zereffa, E. A. Multifunctional application of PVA-aided Zn-Fe-Mn coupled oxide nanocomposite. Nanoscale Research Letters. 16, 1 (2021).
- Shekofteh-Gohari, M., Habibi-Yangjeh, A. Fe3O4/ZnO/CoWO4 nanocomposites: Novel magnetically separable visible-light-driven photocatalysts with enhanced activity in degradation of different dye pollutants. Ceramics International. 43 (3), 3063-3071 (2017).
- Saravanan, R., Gupta, V. K. K., Narayanan, V., Stephen, A. Visible light degradation of textile effluent using novel catalyst ZnO/γ-Mn2O3. Journal of the Taiwan Institute of Chemical Engineers. 45 (4), 1910-1917 (2014).
- Buonsanti, R., Milliron, D. J. Chemistry of doped colloidal nanocrystals. Chemistry of Materials. 25 (8), 1305-1317 (2013).
- Hu, H., He, H., Zhang, J., Hou, X., Wu, P. Optical sensing at the nanobiointerface of metal ion-optically-active nanocrystals. Nanoscale. 10 (11), 5035-5046 (2018).
- Deganello, F., Tyagi, A. K. Solution combustion synthesis, energy and environment: Best parameters for better materials. Progress in Crystal Growth and Characterization of Materials. 64 (2), 23-61 (2018).
- Buonsanti, R., et al. Assembly of ligand-stripped nanocrystals into precisely controlled mesoporous architectures. Nano Letters. 12 (7), 3872-3877 (2012).
- Li, F., Ran, J., Jaroniec, M., Qiao, S. Z. Solution combustion synthesis of metal oxide nanomaterials for energy storage and conversion. Nanoscale. 7 (42), 17590-17610 (2015).
- Williams, T. E., et al. Nearest-neighbour nanocrystal bonding dictates framework stability or collapse in colloidal nanocrystal frameworks. Chemical Communications. 53 (35), 4853-4856 (2017).
- Helms, B. A., Williams, T. E., Buonsanti, R., Milliron, D. J. Colloidal nanocrystal frameworks. Advanced Materials. 27 (38), 5820-5829 (2015).
- Liu, B., et al. Synthesis of ZnO nano-powders via a novel PVA-assisted freeze-drying process. RSC Advances. 6 (111), 110349-110355 (2016).
- Abebe, B., Murthy, H. C. A. Insights into ZnO-based doped porous nanocrystal frameworks. RSC Advances. 12 (10), 5816-5833 (2022).
- LaMer, V. K., Dinegar, R. H. Theory, production and mechanism of formation of monodispersed hydrosols. Journal of the American Chemical Society. 72 (11), 4847-4854 (1950).
- Jun, Y. -. S., et al. Classical and nonclassical nucleation and growth mechanisms for nanoparticle formation. Annual Review of Physical Chemistry. 73, 453-477 (2022).
- Gao, Y., Meng, F., Li, X., Wen, J. Z., Li, Z. Factors controlling nanosized Ni-Al 2 O 3 catalysts synthesized by solution combustion for slurry-phase CO methanation: the ratio of reducing valences to oxidizing valences in redox systems. Catalysis Science & Technology. 6 (21), 7800-7811 (2016).
- Abebe, B., Zereffa, E. A., Murthy, H. C. A. Synthesis of poly(vinyl alcohol)-aided ZnO/Mn 2 O 3 nanocomposites for acid orange-8 dye degradation: Mechanism and antibacterial activity. ACS Omega. 6 (1), 954-964 (2021).
- Kumar, S., Krishnakumar, B., Sobral, A. J. F. N., Koh, J. Bio-based ( chitosan / PVA / ZnO ) nanocomposites fi lm Thermally stable and photoluminescence material for removal of organic dye. Carbohydrate Polymers. 205, 559-564 (2019).
- Dai, Y., et al. Enhanced mechanical, thermal, and UV-shielding properties of poly(vinyl alcohol)/metal-organic framework nanocomposites. RSC Advances. 8 (67), 38681-38688 (2018).
- Munawar, T., et al. Novel tri-phase heterostructured ZnO-Yb2O3-Pr2O3 nanocomposite; structural, optical, photocatalytic and antibacterial studies. Ceramics International. 46 (8), 11101-11114 (2020).
- Mukhtar, F., et al. Enhancement in carrier separation of ZnO-Ho2O3-Sm2O3 hetrostuctured nanocomposite with rGO and PANI supported direct dual Z-scheme for antimicrobial inactivation and sunlight driven photocatalysis. Advanced Powder Technology. 32 (10), 3770-3787 (2021).
- Lachheb, H., et al. Electron transfer in ZnO-Fe 2 O 3 aqueous slurry systems and its effects on visible light photocatalytic activity. Catalysis Science & Technology. 7 (18), 4041-4047 (2017).
- Thommes, M., et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure and Applied Chemistry. 87 (9-10), 1051-1069 (2015).
- Kumar, P., Kim, K. -. H., Kwon, E. E., Szulejko, J. E. Metal-organic frameworks for the control and management of air quality: advances and future direction. Journal of Materials Chemistry A. 4 (2), 345-361 (2016).
- Liu, J., et al. NiO-PTA supported on ZIF-8 as a highly effective catalyst for hydrocracking of Jatropha oil. Scientific Reports. 6, 23667 (2016).
- Fatehah, M. O., Aziz, H. A., Stoll, S. Stability of ZnO nanoparticles in solution. Influence of pH, dissolution, aggregation and disaggregation effects. Journal of Colloid Science and Biotechnology. 3 (1), 75-84 (2014).
- Sigoli, F. A., Davolos, M. R., Jafelicci, M. Morphological evolution of zinc oxide originating from zinc hydroxide carbonate. Journal of Alloys and Compounds. 262-263, 292-295 (1997).
- Wachs, I. E. Raman and IR studies of surface metal oxide species on oxide supports: Supported metal oxide catalysts. Catalysis Today. 27 (3-4), 437-455 (1996).
- Parler, C. M., Ritter, J. A., Amiridis, M. D. Infrared spectroscopic study of sol-gel derived mixed-metal oxides. Journal of Non-Crystalline Solids. 279 (2-3), 119-125 (2001).
- Anžlovar, A., Kogej, K., Crnjak Orel, Z., Žigon, M. Polyol mediated nano size zinc oxide and nanocomposites with poly(methyl methacrylate). Express Polymer Letters. 5 (7), 604-619 (2011).
- Saravanan, R., et al. ZnO/Ag/Mn 2 O 3 nanocomposite for visible light-induced industrial textile effluent degradation, uric acid and ascorbic acid sensing and antimicrobial activity. RSC Advances. 5 (44), 34645-34651 (2015).
- Yang, G., Yan, W., Wang, J., Yang, H. Fabrication and formation mechanism of Mn 2 O 3 hollow nanofibers by single-spinneret electrospinning. CrystEngComm. 16 (30), 6907-6913 (2014).
- Liu, Y., et al. A magnetically separable photocatalyst based on nest-like γ-Fe 2 O 3 /ZnO double-shelled hollow structures with enhanced photocatalytic activity. Nanoscale. 4 (1), 183-187 (2012).
- Hu, Y., et al. A microwave-assisted rapid route to synthesize ZnO/ZnS core-shell nanostructures via controllable surface sulfidation of ZnO nanorods. CrystEngComm. 13 (10), 3438-3443 (2011).
- Zhang, J., et al. Synthesis and gas sensing properties of α-Fe 2 O 3 @ ZnO core-shell nanospindles. Nanotechnology. 22 (18), 185501 (2011).
- Penn, R. L. Imperfect oriented attachment: Dislocation generation in defect-free nanocrystals. Science. 281 (5379), 969-971 (1998).
- Zhang, J., Huang, F., Lin, Z. Progress of nanocrystalline growth kinetics based on oriented attachment. Nanoscale. 2 (1), 18-34 (2009).
- Zeng, Z., et al. A fluorescence-electrochemical study of carbon nanodots (CNDs) in bio- and photoelectronic applications and energy gap investigation. Physical Chemistry Chemical Physics. 19 (30), 20101-20109 (2017).
- Zhai, T., et al. Controllable synthesis of hierarchical ZnO nanodisks for highly photocatalytic activity. CrystEngComm. 14 (5), 1850-1855 (2012).
- Li, N., et al. Efficient removal of chromium from water by Mn3O4 @ZnO/Mn3O4 composite under simulated sunlight irradiation: Synergy of photocatalytic reduction and adsorption. Applied Catalysis B: Environmental. 214, 126-136 (2017).
- Abebe, B. Polymer assisted colloidal nanocrystal framework synthesis: Sol-gel approach. Materials Research Express. 8 (12), 125005 (2021).
- Jiamprasertboon, A., et al. Heterojunction α-Fe2O3/ZnO films with enhanced photocatalytic properties grown by aerosol-assisted chemical vapour deposition. Chemistry - A European Journal. 25 (48), 11337-11345 (2019).
- Mukhtar, F., et al. Dual S-scheme heterojunction ZnO-V2O5-WO3 nanocomposite with enhanced photocatalytic and antimicrobial activity. Materials Chemistry and Physics. 263, 124372 (2021).
- Marschall, R. Semiconductor composites: Strategies for enhancing charge carrier separation to improve photocatalytic activity. Advanced Functional Materials. 24 (17), 2421-2440 (2013).
- Beranek, R. (Photo)electrochemical methods for the determination of the band edge positions of TiO 2-based nanomaterials. Advances in Physical Chemistry. 2011, 786759 (2011).
- Hoffmann, M. R., Martin, S. T., Choi, W., Bahnemann, D. W. Environmental applications of semiconductor photocatalysis. Chemical Reviews. 95 (1), 69-96 (1995).
- Wu, Y., Wang, D., Li, Y. Understanding of the major reactions in solution synthesis of functional nanomaterials. Science China Materials. 59, 938-996 (2016).
- Xia, Y., Xiong, Y., Lim, B., Skrabalak, S. E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics. Angewandte Chemie. 48 (1), 60-103 (2008).
- Kim, S. J., Yoon, S., Kim, H. J. Review of solution-processed oxide thin-film transistors. Japanese Journal of Applied Physics. 53, (2014).
- Zhang, J., Guo, Q., Liu, Y., Cheng, Y. Preparation and characterization of Fe2O3/Al2O3 using the solution combustion approach for chemical looping combustion. Industrial & Engineering Chemistry Research. 51 (39), 12773-12781 (2012).
- Novitskaya, E., Kelly, J. P., Bhaduri, S., Graeve, O. A. A review of solution combustion synthesis: an analysis of parameters controlling powder characteristics. International Materials Reviews. 66 (3), 188-214 (2021).
- González-Cortés, S. L., Imbert, F. E. Fundamentals, properties and applications of solid catalysts prepared by solution combustion synthesis (SCS). Applied Catalysis A: General. 452, 117-131 (2013).
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可探索更多文章
This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。