Method Article
Randomized, Triple-Blind, and Parallel-Controlled Trial of Transcranial Direct Current Stimulation for Cognitive Rehabilitation after Stroke
* These authors contributed equally
In This Article
Summary
This study presents a novel transcranial direct current stimulation (tDCS) protocol combined with cognitive stimulation to address post-stroke hemispatial neglect. Initial data from a pilot patient ensure the procedure's feasibility and suggest potential efficacy, providing a foundation for a future parallel, triple-blind, controlled clinical trial.
Abstract
Right hemisphere stroke frequently results in hemispatial neglect, a disabling condition that can significantly impede the recovery process. The chronic presence of neglect has been linked to poorer outcomes in both cognitive and motor domains. As an adjunct to conventional neuropsychological interventions, transcranial direct current stimulation (tDCS)-a noninvasive technique that modulates neural excitability through low-intensity electrical currents-has gained attention for its potential to enhance cortical plasticity and support functional improvement in affected individuals.
In this study, we propose a combined intervention protocol aimed at reducing symptoms of post-stroke hemispatial neglect. It consists of a cathodal tDCS protocol combined with a computerized neuropsychological rehabilitation program specifically designed for the rehabilitation of hemispatial neglect.
The neuromodulation strategy is to reduce the hyperactivation of the undamaged hemisphere based on the interhemispheric rivalry model. The intervention consists of 2 weeks, 10 sessions (from Monday to Friday), 45 min each, of tDCS and conventional cognitive stimulation concurrently applied. The tDCS is applied by an 8-channel high definitiontDCS (HD- tDCS) device for 20 min and at 2 mA of intensity. The cathode is positioned over the left posterior parietal cortex (P3 following 10/20 system for electroencephalogram [EEG] electrode placement), and return electrodes are placed at C3, CP5, CP1, Pz, PO3, PO7, and P7. A neuropsychological and functional assessment was carried out at baseline and after the end of the intervention.
The primary aim of the present study is to describe the protocol for a parallel, randomized, triple-blind experimental design. To ensure the feasibility of the protocol and its potential efficacy, a comprehensive description of the procedures applied to a single pilot participant is provided.
Incorporating tDCS neuromodulation strategies into cognitive rehabilitation processes may lead to shortened intervention times and improve the functional status and quality of life of patients.
Introduction
Stroke is the most common cause of disability in the world in adults and the second cause of death after ischemic heart disease1. Most patients who have survived a stroke develop very heterogeneous clinical conditions and different degrees of disability. Between 55% and 75% of stroke patients have motor limitations that persist 6 months after the injury2. In addition to the physical consequences, cognitive alterations are very frequent3. These deficits negatively affect the performance of activities of daily living, limiting the functional independence and the quality of life of patients and relatives4,5. Hemispatial neglect is among the most common attentional impairments following a stroke, occurring in approximately 25% to 50% of cases6,7,8 and rising to as much as 80% in individuals with right-hemisphere strokes9,10.
Hemispatial neglect implies a difficulty in attending the contralateral hemifield to the injured area, being this inattention allocentric (omitting objects located in the left half of the space) or egocentric (patient does not attend to left parts of his/her own body). Functionally, neglect generates severe difficulties in the independence of the patient, both in basic (e.g., grooming, clothing, eating, etc.) and instrumental activities of daily living (e.g., money management, public transport, or independent walking). Moreover, the presence of this alteration has been associated with longer hospitalization and rehabilitation times, higher risk of falls, poor motor recovery, and lower probability of returning home after discharge from hospital11,12.
Several strategies have been implemented to treat hemispatial neglect. Within traditional rehabilitation approaches, we can distinguish the top-down and the bottom-up approaches. The main difference between them is the level of active participation and awareness of the person in tasks. Within these approaches, the most widely used procedures to date have been visual scanning training and prismatic adaptation, respectively13. Other rehabilitation techniques in hemispatial neglect with wide use central location, optokinetic, caloric, and vestibular stimulation, neck vibration, and pharmacological treatments13,14,15,16. However, these treatments have some limitations: the duration of their results is very limited, and they have low applicability in the acute or subacute phases because the severity of the patients in these phases interferes with their collaboration in the activities to be performed17.
Transcranial direct current stimulation (tDCS) is a noninvasive safe neuromodulation technique able to modify cortical activity by inducing a weak electric current into the brain that changes the cortical activity, and it can be used to complement neuropsychological rehabilitation for hemispatial neglect. tDCS modulates spontaneous neuronal activation in response to inputs from other brain areas. Furthermore, tDCS induces plastic synaptic changes that resemble long-term potentiation (LTP) or long-term depression (LTD) and even last beyond the duration of stimulation18.
By means of tDCS, cortical activity can be modulated by applying a very low-intensity electric current that flows from the anode to the cathode. tDCS modulates brain activity by influencing the threshold of the action potential, increasing or decreasing it, but without causing action potentials18. In general, the anode induces an increase in the excitability of the brain region on which it is located, while the cathode induces cortical inhibition. This technique does not have a high spatial resolution, but this limitation has been overcome by the appearance of new tDCS devices called multisite or high definition (HD- tDCS). These devices allow different electrode configurations, such as forming a cathodal ring around the anode (or vice versa) in order to increase or decrease cortical excitability in a specific brain area. The cathode ring acts in a similar way to the return electrodes, limiting the stimulation area; in this way, a more focal stimulation is achieved. tDCS has proven effective as a therapeutic approach for motor recovery after stroke19, and there is some scientific literature with promising results in the rehabilitation of hemispatial neglect20.
The most accepted hypothesis of hemispatial neglect argues that it could be explained based on the hemispheric rivalry model, proposed by Kinsbourne in 197721,22. According to this approach, in the basal state, both hemispheres are constantly inhibiting each other in a reciprocal manner; hemispatial neglect is caused by an imbalance between them. After an injury, the damaged hemisphere is not able to effectively inhibit the activity of the preserved hemisphere. This results in pathological hyperactivity of the healthy hemisphere due to the absence of inhibition exerted by the damaged one, which reduces, even more, the neural activity of the affected hemisphere because of the increased inhibition exerted over it23. Therefore, the dysfunction that underlies hemispatial neglect is caused by both hypoactivity of the damaged hemisphere and hyperactivity of the intact one24.
With this model as a theoretical background, different noninvasive brain stimulation strategies aimed to improve hemispatial neglect symptoms are proposed. These strategies are addressed to decrease the hyperactivity of the healthy hemisphere, increase the activity of the injured hemisphere, or a combination of both25,26.
Several studies have shown the potential of tDCS in reducing hemispatial neglect symptomatology by applying both anodal17,27,28,29 and cathodal17,29 tDCS in the injured or undamaged hemisphere, respectively, or a combination of both28,30,31,32. Despite promising results, more empirical evidence is needed to know the exact parameters of tDCS to achieve optimal results, which is essential to know if focal tDCS is more effective than conventional tDCS montages. To our knowledge, all the previous research has been developed using conventional tDCS, with the present study being the first to use HD-tDCS for hemispatial neglect rehabilitation.
Interventions based on noninvasive brain stimulation constitute a very promising clinical approach given the results up to date and the limited adverse effects according to different meta-analyses and reviews33,34,35,36,37. In addition, tDCS is a highly safe, portable, and low-cost technique, which is why its use has increased as a priority in clinical and research settings. Also, its easy assembly and portability allow the device to be used simultaneously with the performance of any other activity, such as physical, cognitive rehabilitation, or functional activities. Thus, more controlled, blinded, randomized studies with larger sample sizes are warranted to validate tDCS protocols that enhance the effects of conventional intervention approaches.
Protocol
This project has been approved by the Clinical Research Ethics Committee of 12 de Octubre Hospital (ref. Nº CEIm: 19/180), and it is registered at www.clinicaltrials.gov (ID: NCT04458974). The researchers agree to respect all the established current legislation regarding clinical research and data protection (WMA Declaration of Helsinki, 2004; Regulation (EU) 2016/679 and organic law 3/2018 on personal data protection; Law 41/2002 on patient autonomy). In accordance with Regulation (EU) 2016/679 on personal data protection, any data collected from the participants will be treated with strict confidentiality. The tDCS protocol follows the international safety guidelines for tDCS38.
NOTE: The primary aim of the present study is to describe a tDCS intervention protocol for a parallel, randomized, triple-blind clinical trial. To achieve this, a comprehensive description of the procedures is provided, and the results of a pilot participant application are shown in this paper. The intervention protocol consists of a 10-session program combining cathodal tDCS (20 min, 2 mA) with a computerized neuropsychological rehabilitation program designed to improve hemispatial neglect. Neuropsychological and functional assessments are performed at baseline and after the end of the intervention. Figure 1 shows the timeline of the protocol. The figure shows the baseline assessment, detailed description of the intervention, and post-intervention assessment of the study. Patient participation was voluntary after being informed about the purpose of the study and signing a written informed consent form. The participant may withdraw from the study at any time. The participant of this study meets all the inclusion and exclusion criteria outlined in Table 1.
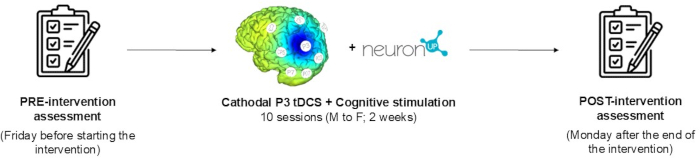
Figure 1: Protocol timeline. All stages of the study are described: baseline assessment, detailed description of the intervention, and post-intervention assessment. Please click here to view a larger version of this figure.
1. Inclusion and exclusion criteria
- Ensure that the pilot participant in this study meets the following inclusion and exclusion criteria (Table 1).
| Inclusion criteria: |
| Hemorrhagic or ischemic stroke in the right hemisphere |
| Stroke 3 to 12 months since the event (regardless of whether or not they have received prior rehabilitation) |
| 18 to 89 years old |
| Neuroimaging study |
| Absence of previous strokes |
| Functional capacity that allows the patient to remain seated and active for one hour (Barthel Index score greater than 5 in the item of transfers between chair and bed; this item can be scored from 0 to 15, being 0 totally dependent and 15 totally independent). |
| Right-handed manual dominance |
| Neglect scores on at least two of the tests administered for the assessment of visuospatial neglect |
| Signature of informed consent by the patient or his/her legal guardian |
| Exclusion criteria: |
| Dermatological problems (psoriasis, dermatitis on the scalp or face) |
| Presence of implants or metal parts in the head excluding fillings. |
| Pacemakers, medication pumps, stimulators (vagal, cerebral, transcutaneous), ventriculoperitoneal shunts, or aneurysm clips. |
| Presence of previous strokes |
| Neurological disease other than stroke described in the inclusion criteria |
| Severe cognitive impairment assessed using the Mini-mental state examination (MMSE) (Folstein, 1975), excluding patients with scores under 24 (the score of MMSE are between 0 and 30, being 0 severe cognitive impairment and 30 no cognitive impairment) |
| Significant language difficulties that do not allow a proper understanding of activities or severely limit expression |
| History of alcohol or drug abuse |
| Moderate or severe active depression |
| Uncontrolled medical problems (pathologies in acute phase without medical or pharmacological treatment with proven efficacy or pathologies with imminent life risk) |
| Pregnancy or suspected pregnancy that will be checked by pregnancy test at the beginning of the study in patients of childbearing age and with the recommendation of the use of contraceptive methods until the end of the intervention |
Table 1: Inclusion and exclusion criteria. The pilot participant of this study meets all the inclusion and exclusion criteria described in this table.
2. Materials
NOTE: All the materials used in all phases of the study are meticulously described.
- tDCS device kit
- For the application of tDCS, use an 8-channel HD-tDCS device and its recommended electrodes (see Figure 2). To facilitate easier assembly, place a plastic base in the desired location on the neoprene cap (following the 10/20 EEG system). Once placed, apply the conductive gel and subsequently assemble the electrode.
- Computerized neurorehabilitation platform
NOTE: To carry out the cognitive stimulation, an online computerized neurorehabilitation platform is used (see Figure 3). The platform allows the design and application of every session of the intervention program, adapting the difficulty of the tasks individually.- Create a new user for each patient by clicking on user management and adding a new user. Name using a study code to personalize the cognitive program individually.
- To design the tasks, access the work area/sessions/digital sessions and click on the date on which the session will take place.
- Insert the name of the session, for example, session 1. Select an icon and a color for that session and click the Create button.
- Click on the activities to include in the program.
- Check that the tasks for each session appear on the left side of the screen.
- In each of them, program the following parameters: Time (7 min) and start at the last result (only indicate YES in sessions 3-10).
- Indicate to save.
- Repeat steps 2.2.3-2.2.7 for the design of each of the 10 sessions of the cognitive stimulation program.

Figure 2: tDCS device kit. (1) Neoprene cap, (2) tDCS device, (3) Electrodes, (4) Cables, (5) Ear clip, (6) Conducting gel; (7) Syringe to administer the conductive gel under the electrodes. Please click here to view a larger version of this figure.
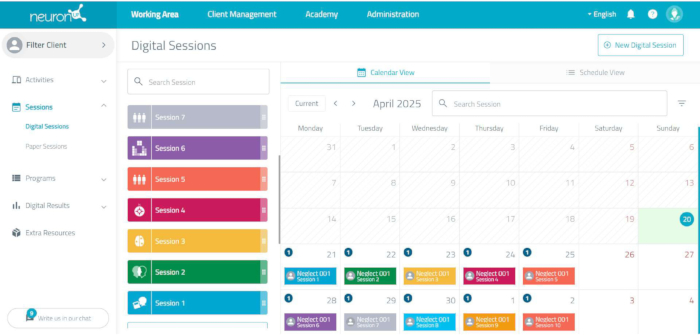
Figure 3: The neurorehabilitation platform session scheduling. By clicking on each session, the tasks are displayed, including the duration of each task and the total duration of the session. Please click here to view a larger version of this figure.
3. Description of the assessment protocol: Pre-intervention neuropsychological and functional assessment:
NOTE: Once the participant signed the informed consent, a neuropsychological and functional assessment is conducted. The evaluation takes place on the Friday before the start of the stimulation program and lasts 50 min. During this session, general cognitive performance is assessed, and neuropsychological tests focused on evaluating attentional processes and hemispatial neglect are administered, along with functional scales. In an information session prior to the first evaluation, the participant is provided with all necessary details about the study's objective, procedure, session duration, and potential adverse effects. Signed consent is obtained before proceeding with the evaluation and intervention. The participant is also informed of the option to withdraw from the study at any time.
- Mini mental state examination (MMSE) administration (5 min)
NOTE: A simple structured scale. It scores a maximum of 30 points, and the items are grouped into 5 sections that assess orientation, immediate memory, attention and calculation, deferred memory, language, and construction. The scores range from 0-30 points, with the cut-off point at 24; the scores below 24 indicate cognitive impairment.- Explain the test to the participant and have the participant follow the test instructions. Check that the total score is ≥24 points to continue.
- Line bisection test administration (5 min)
NOTE: Eighteen lines are presented in an A4 size sheet organized in 3 sets of 6: one set located mainly to the left of the paper, one in the center of the paper, and one mainly to the right of the paper. Patients are asked to mark the center points of each line. The deviation of the patients' mark from the true center of the line is measured for each line and averaged for the 18 lines.- Place the task centered in front of the participant, indicating on the sheet the right and left sides of the participant. Explain the test to the participant and have the participant follow the test instructions.
- Bells test administration (5 min)
NOTE: An instrument aimed at evaluating horizontal visual scanning in the presence of distractors. The task involved identifying bell-shaped figures arranged in a semi-random manner. These were displayed in seven vertical columns, each containing five bells. Regarding their placement on the sheet, three columns appeared on the left, one in the center, and three on the right.- Place the task centered in front of the participant, indicating on the sheet the right and left sides of the participant. Explain the test to the participant and have the participant follow the test instructions.
- Picture drawing subtests from Barcelona test administration (5 min)
NOTE: It consists of a copy of 6 figures: circle, square, triangle, cross, cube, and house. The time of execution is controlled. Each figure is scored as follows: 3 points for perfect reproduction, 2 points for discrete alteration, 1 point for moderate alteration, 0 points for serious alteration. The maximum score is 18. It is rewarded with 3, 2, or 1 point, depending on the time spent in the execution.- Place the task centered in front of the participant, pointing to the pictures that appear. Explain the test to the participant and have the participant follow the test instructions.
- Cancellation subtests from Barcelona test administration (5 min)
- Place the task centered in front of the participant, indicating on the sheet the right and left sides of the participant. Explain the test to the participant and have the participant follow the test instructions.
- Digit span forward and backward administration (5 min).
- Explain the test to the participant and have the participant follow the test instructions.
- Manage the direct digit task first. Secondly, administer the inverse digits task next.
- Brief test of attention (BTA) administration (5 min)
- Administer verbally the first part of the task (count the numbers that appear in the sequence). Immediately after, apply the second part of the test (count the letters that appear in the sequence)
- Do not allow the use of notes or counting on fingers.
- Face test administration (5 min)
- Present the face test sheet. Explain the test to the participant and have the participant follow the test instructions.
- Activate the stopwatch and finish the task after 3 min. Count the number of correctly marked stimuli.
- Motor-free visual perception test (MVPT-4) administration (15 min)
NOTE: The 36-element multiple-choice test assesses 5 subdimensions of visuospatial neglect: visual discrimination, figure-bottom discrimination, spatial relationship, visual closure, and visual memory. Different parameters are used to quantify the behavior of the left response (left/right response behavior, gross score, left/right performance, and time of processing of visual perception). Scores for left-sided responses range from 0 to 21, with lower values indicating more severe visuospatial neglect.- Place the homework notebook in front of the patient. Explain the test to the participant and have the participant follow the test instructions.
- Functional scales
NOTE: Conduct patient and family member/caregiver interviews to know the patient's functional status and the impact of neglect on activities of daily living.- Administer the Barthel Index (5 min) scale to the family member or primary caregiver and record responses on the answer sheet.
NOTE: The Barthel index measures the capability of a person to perform ten basic activities of daily living, obtaining a quantitative estimate of their degree of independence. - Administer the Catherine Bergego Scale (CBS) (5 min) to the family member or primary caregiver and record responses on the answer sheet.
NOTE: This questionnaire relies on direct observation of the patient's performance in 10 clinically simulated real-life situations, including tasks like grooming, dressing, and wheelchair use. The patient's assessment is compared with that of a family member / primary caregiver. This questionnaire yields a score between 0 and 30, where higher scores reflect greater severity of visuospatial neglect. .
- Administer the Barthel Index (5 min) scale to the family member or primary caregiver and record responses on the answer sheet.
4. Description of the intervention protocol
- Intervention device configuration
NOTE: Set the parameters of HD-tDCS stimulation in the software. Days before starting the intervention, the device must be programmed with the parameters to be applied in the intervention, i.e., duration, intensity, polarity, condition (active vs. sham), montage, type of electrodes, stimulation site, returned electrodes positions, and percentage of electric return to each one (see point 5). Check that the device has enough battery to complete the entire session.- Open the software, select Protocol Editor, and then add a new protocol.
- Enter the name of the protocol. Set the duration of the ramp: Steps: (left).
- Confirm the ramp and the total stimulation time. Indicate Ramp-Up: 30 and Ramp-down: 30.
- In the total stimulation duration section, indicate 20 min. Set the stimulation and electrode settings: Design (right).
- Set the polarity of the stimulation in cathodal. Set up each electrode independently. Start with the active electrode, P3.
- Select electrode P3 and drag it to the box on the right. Select stimulation and cathodal. Select 2000 µA.
- Select and drag to the left, space the rest of the electrodes one by one. In these cases, they are all return electrodes and do the same action, indicating the percentage of return in each electrode.
NOTE: It is important to note that the sum of the return percentage must add up to 100. In this study, the return percentages were CP1, PO3, PO7, P7, CP5, C3: 10% and PZ: 40% (see Figure 4 and Figure 5). This montage is designed to reduce the pathological hyperactivation of the posterior parietal cortex in the hemisphere contralateral to the lesion. - Click on the Finish button once all steps are completed.
- Patient pre-intervention assessment
- General state assessment.
- Before and after each tDCS session, assess the general condition by asking the following questions: Are you feeling well? Do you have a headache? Do you have neck pain? Did you drink alcohol yesterday or today? Did you use drugs yesterday or today? Have you consumed coffee, tea, or chocolate in the last 5 h? Did you sleep well last night? When was the last time you ate in hours?
- Assess the visual analog scales (VAS) for fatigue and mood.
- Assess the level of mental fatigue and mood using two self-administered VAS. In response to these questions, ask the participant to provide a score between 1 and 10, referring to how they feel regarding the questions: How tired are you right now?, where 1 means not tired at all, 10 means very tired, and How is your mood right now?, where 1 means very sad and 10 means very happy.
- General state assessment.
- High-definition tDCS montage
- Before starting the assembly of the tDCS device, check that all the necessary materials are available and that the tDCS device has enough battery to perform the stimulation session.
- Plastic lower part of the electrode placement
- Select in the neoprene cap the following positions: place the active cathode electrode at P3; place the return electrodes at C3, CP5, CP1, Pz, PO3, PO7, and P7 (see Figure 5 and Figure 6).
- Place the lower part of the electrode in each of the selected areas of the cap. Put the cap on the participant's head, adjusting Cz based on the measurements previously taken.
- tDCS neoprene cap placement
- Seat the participant in a comfortable position in a chair.
- Put on the neoprene cap (see Figure 6), selecting the size that best fits the subject's head. Avoid selecting a big cap since It must be tight to the head. Adjust the cap with the headband until it fits snugly on the head.
- Locate the Cz point and the stimulation zones of the protocol.
- Measure the distance between nasion and inion and between the preauricular points, using a meter. Locate the Cz point at the midpoint of both locations.
- Electroconductive gel placement
- Separate the hair located under the electrode from the place of stimulation. Ensure good contact between the electrode and the skin; use an elongated object that allows the hair to be removed from inside the electrodes without modifying the placement of the cap have been previously measured.
- Ensure the scalp is dry without any other added preparation. Remove hairpins or other metal elements (headbands, barrettes, etc.).
- Never clean the skin with alcohol since it could produce abrasion with tDCS.
- Introduce, employing a syringe with a final plastic tip, a sufficient quantity of electroconductive gel at the base of each electrode.
CAUTION: Only electroconductive gel may be used; never use water or saline solution in this type of electrode because it may cause abrasion. - Remove as much hair as possible with the help of the plastic tip of the syringe.
- Impedance checking
NOTE: The Impedance Check button measures the impedance of all active and return channels prior to stimulation. It must be used before initiating any stimulation protocol. Impedance levels are displayed as color-coded bars beneath each stimulation channel icon, with green indicating acceptable values. The stimulation program can only begin once all channels show green. During stimulation, impedance is monitored every second, and if any electrode exceeds 20 kW at any moment, the protocol is automatically interrupted.- Turn on the device. Open the tDCS device software on the computer.
- Select the connection used with the device. Select the scan for device option.
- Select Check Impedance.
- If all electrodes appear in green, press the Play button and the stimulation program will start.
- If, after completing the impedance check, any electrode appears red, reapply gel to that electrode, remove any hair, and recheck the impedance until all electrodes remain green.
- Computerized neurorehabilitation platform
NOTE: Design the 10 intervention sessions in advance on the neurorehabilitation platform and prepare the platform for it (see point 2.2). Each session consists of four tasks, each lasting 7 min. The tasks included in each session are shown in Table 2. When scheduling each session, it is necessary to select among the parameters of each task: time duration of each task: 7 min, total duration of the session (28 min), language, include user evaluation after each activity, include confirmation of the end of each activity at the end of each task, and absence of the continuous button in each task, since tasks must be done sequentially one after another (Figure 7).- Access the computerized neurorehabilitation platform.
- Access the previously planned session (see step 2.2) and press the Start button. Present four different stimulation activities of 7 min each sequentially.
NOTE: The cognitive stimulation session has a total duration of 30 min. After the total simulation time (30 min), the activities stop. Cognitive stimulation continues for 5 min more after the end of the tDCS stimulation.
- tDCS stimulation program (20 min)
- Activate the start of tDCS stimulation by pressing the Play button on the tDCS control software 4 min and 20 s after the start of the computerized neurorehabilitation task.
- After 30 s of ascending ramp, the active stimulation or sham starts. Apply tDCS stimulation (20 min) concurrently to the computerized neurorehabilitation task. After 20 min., a descent ramp begins and lasts for 30 s. After this time, tDCS stimulation stops.
- Patient post-intervention assessment.
- Side effects questionnaire.
- Apply an adaptation of the Questionnaire of Sensations Related to Transcranial Electrical Stimulation39 (Table 3) with 9 questions about the presence of various symptoms such as headache, burning on the scalp, or itching or tingling sensation under the electrodes.
- In each item, rate the severity of the symptom on a scale of 1-4 and its relationship with tDCS on a scale of 1-5 (see Table 3).
- Visual analog scales for fatigue and mood
- Administer the same scale used before the stimulation session to evaluate fatigue and mood after the intervention (see step 4.2.2.).
- Side effects questionnaire.
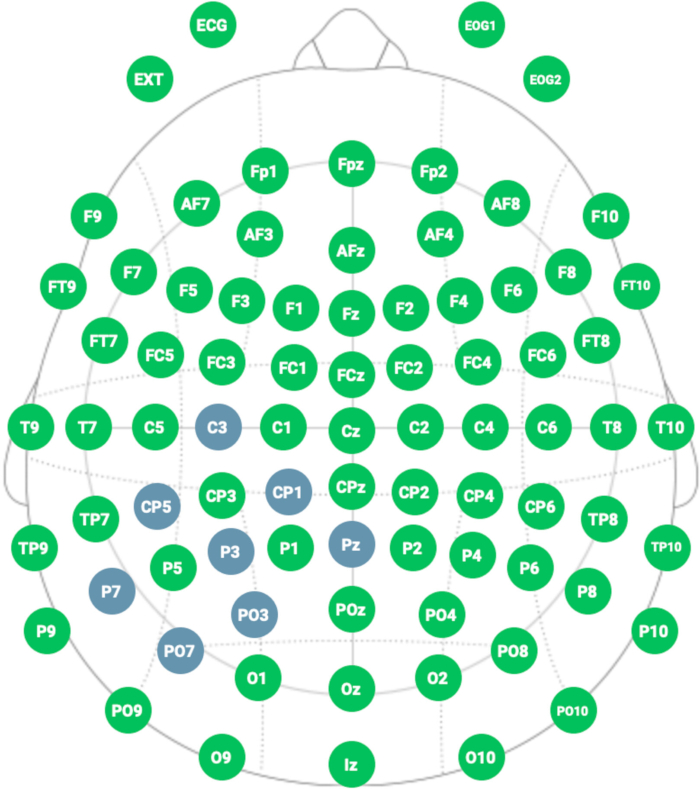
Figure 4: Representative HD- tDCS montage. The blue color shows the location of the electrodes following the international 10-20 system for electrode placement. Please click here to view a larger version of this figure.
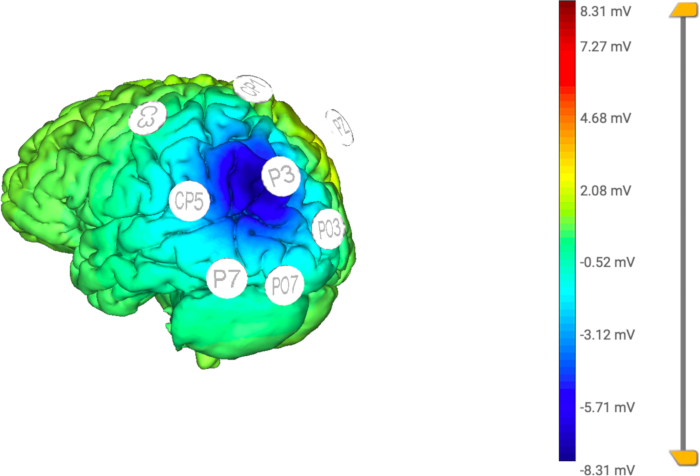
Figure 5: Computational model of the applied HD- tDCS. The distribution of the electric current is focused in the central (active) electrode (P3), limiting the stimulation to a specific area by the return electrodes (C3, CP5, CP1, Pz, PO3, PO7, P7). Please click here to view a larger version of this figure.

Figure 6: Neoprene cap for HD- tDCS stimulation. The cap has holes to place electrodes following the 10/20 classification system for EEG. Please click here to view a larger version of this figure.

Figure 7: Scheduling tasks. Parameters to be selected when programming each task in the Computerized Neurorehabilitation platform. Please click here to view a larger version of this figure.
| Block | Session | Neurorehabilitation Platform Tasks | Duration |
| I | 1, 3, 6, and 8 | · Hidden letters | 7 min each of the tasks. |
| · Sum of figures | |||
| · Letter soup | |||
| · Find matching shapes | |||
| II | 2, 4, 7, and 9 | · Copying letter matrices | |
| · The little wolverine | |||
| · Finding missing numbers | |||
| · Comparing texts | |||
| III | 5 and 10 | · Text comparison | |
| · Sum of figures | |||
| · The little glutton | |||
| · Search for consecutive stimuli |
Table 2: Tasks included in each of the 10 intervention sessions. All tasks are included in the Computerized Neurorehabilitation platform.
| Do you have any of the following sensations or symptoms? | Grading of severity (1–4) | If present: relationship with tDCS? |
| 1-Absent | 1-None | |
| 2-Mild | 2-Remote | |
| 3-Moderate | 3-Possible | |
| 4-Severe | 4-Probable | |
| 5-Definitive | ||
| Headache | ||
| Neck or cervical pain | ||
| Scalp pain | ||
| Scalp burn | ||
| Sensations under the electrode (tingling, itching, burning, pain) | ||
| Skin redness | ||
| Numbness | ||
| Concentration problem | ||
| Sharp mood swings | ||
| Others (specify) | ||
| Additional comments | ||
Table 3: tDCS side effect questionnaire. A list of adverse effects that may appear after the application of the stimulation is provided. The presence or absence of each of these effects is recorded immediately after the application of the stimulation. Adaptation of The Questionnaire of Sensations Related to Transcranial Electrical Stimulation39.
5. tDCS removal
- Once the tDCS stimulation program and the computerized neurorehabilitation tasks have finished, close the tDCS software.
- Disconnect the tDCS device.
- Remove the cable that connects the tDCS with the electrodes.
- Remove the cap from the patient's head.
- Remove the electrodes from the neoprene cap.
- Clean the patient's hair.
- After removing the neoprene cap, remove the remaining gel with the help of a paper/towel and wash with water.
- Clean the tDCS neoprene cap and electrode.
- Wash the cap with water to remove the remaining gel after each session and let it dry.
- Wash the electrodes with water, avoid rubbing them, and gently dry them with a dry cloth. Never use soap, alcohol, or other products for cleaning the electrodes.
6. Post-intervention neuropsychological and functional assessment
- On Monday, after completing the intervention, follow the same procedure as in the pre-evaluation, administering the same evaluation tasks and questionnaires (see section 3).
Results
The primary aim of the present study is to describe a tDCS intervention protocol for a parallel, randomized, triple-blind clinical trial. To study the feasibility of the intervention, the protocol was applied to a single participant; the results are shown in this paper.
We applied the complete intervention protocol to a 57-year-old male with a high educational level (Journalism Degree) who suffered a stroke in the right basal ganglia nine months before and met all the inclusion criteria for participation in the study.
The pre- and post-intervention results (see Table 4 and Figure 8) are shown for all tasks administered. In the post-intervention assessment quantitative changes were observed in 6 of the 13 measured variables, 4 of which are directly related to neglect.
| Assessments | ||
| Pre-intervention | Post-intervention | |
| MMSE test | 29 | 29 |
| Bells test | 21 | 25* |
| Cancellation test | 24 | 28* |
| Drawings test | 12 | 12 |
| Bisection test | 19.95 | 3.47* |
| BTA test | 11 | 9 |
| Faces Hits | 11 | 9 |
| Faces Errors | 3 | 2* |
| Direct Digits test | 11 | 10 |
| Inverse Digits test | 9 | 8 |
| Motor Free | 26 | 29* |
| Barthel test | 25 | 30* |
| CBS | 20 | 20 |
Table 4: Results of pre- and post-intervention assessments. The results of the neuropsychological and functional assessment before and after the intervention are shown in direct scores. *Quantitative improvements in performance in post-intervention assessment compared to baseline. **Values further away from score 0 indicate worse performance and greater neglect.
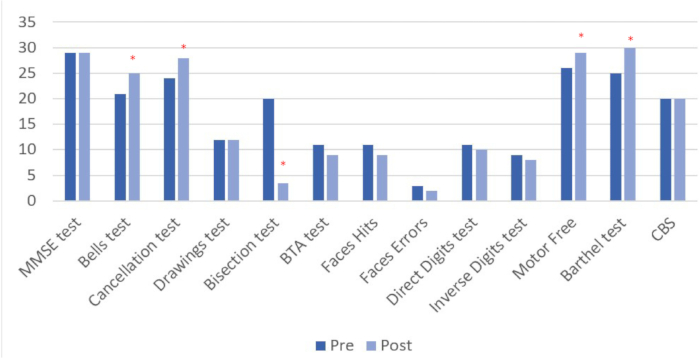
Figure 8: Pre and post intervention assessment results. Higher values indicate positive change, except in the line bisection test, where improvement is represented by lower scores. Results are shown in direct scores. *Quantitative improvements in performance in post-intervention assessment compared to baseline. Please click here to view a larger version of this figure.
The percentage improvement between pre- and post-treatment assessment was calculated. Clinical improvement was observed in the specific hemispatial neglect tests: bells test, cancellation test, line bisection, visual motor-free perception test, and Barthel index scale. On the other hand, negative changes were observed in other attentional tasks (digit test, brief test of attention, face test). Finally, no changes appeared in the MMSE, the copy of drawings, or the functional Catherine Bergego Scale (CBS) (See Figure 9).
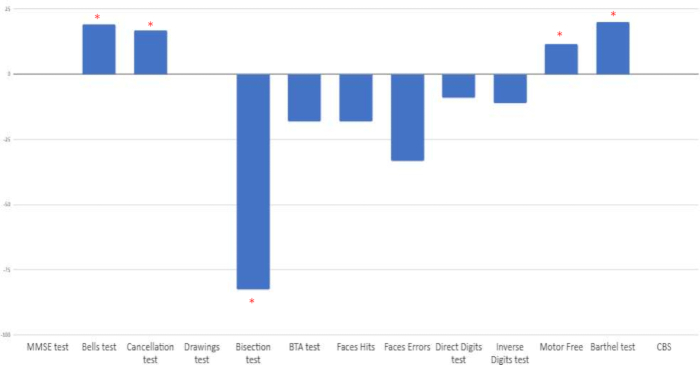
Figure 9: Percentage of change between baseline and post-intervention assessment. Results are shown in percentages. Positive scores indicate positive change, except in the line bisection test, where improvement is represented by negative scores. *Percentage of positive improvement when comparing pre- and post-intervention ratings. Please click here to view a larger version of this figure.
The study procedures were carried out in spacious rooms equipped for the correct execution of the evaluation and intervention sessions and compliance with safety and hygiene measures.
As for the patient, he did not manifest fatigue in any of the pre- and post-evaluation sessions, so it was not necessary to rest in any of them. The therapy was assessed by the patient as entertaining and stimulating, which improved adherence to treatment and active collaboration throughout the procedure. Therefore, it is considered that the protocol would have high feasibility, and we will continue the study following the established procedure.
In terms of discomfort and side effects, the participant did not experience any moderate or severe side effects related to the application of tDCS.
Discussion
Hemispatial neglect is a frequent cognitive consequence of stroke, and when it persists, it tends to negatively impact the effectiveness of the rehabilitation process. The efficacy and efficiency of the available therapeutic approaches can be improved by including noninvasive brain stimulation techniques in neurorehabilitation, looking for a synergistic effect 40,41. Thus, by means of tDCS, we can boost the effectiveness of conventional intervention, achieving greater recovery, shorter rehabilitation times, and better functional outcomes in the rehabilitation of the stroke patient compared with conventional intervention in isolation. Research on the potential of tDCS in neurological and psychiatric disorders has exponentially increased in the last decade42,43,44,45,46,47,48,49.
In addition, the cost of tDCS is affordable, and the device is portable, which makes it highly scalable, allowing its application in both outpatient clinics and hospital settings, with the required professional training50.
We have found an improvement after the treatment in four of the thirteen tests administered (bells test, cancellation test, line bisection, visual motor-free perception test). The tests where we have observed these positive changes are related to the performance associated with hemispatial neglect. On the other hand, stabilization has been observed in the performance of some tests related to general cognitive performance, attentional processes and/or working memory (MMSE, drawings, CBS). A reduction has been observed in the performance of some other tasks (BTA, Faces, direct and inverse digits).
Regarding functional scales, there was evidence of improvement, reported by the primary caregiver and assessed by the Barthel Index scale. The CBS functional scale, which is directly related to the impact of neglect on daily life, was also administered, and, in this case, no change was evidenced, remaining stable with respect to the previous assessment. In this study, we found the benefits of the combined treatment for some cognitive domains but not for others. These findings are consistent with the idea that the treatment could be more beneficial for certain attentional domains51,52,53,54,55,56,57. Some studies show how specific tDCS protocols induce lasting alterations in cortical excitability and activity58. In order to be able to analyze the maintenance of the changes beyond a week, it would be advisable to carry out a new assessment after a longer period of time53,54,55,56,57.
High-definition or high-resolution tDCS, used in this study, is a technically enhanced version of tDCS that allows increasing the focality of the stimulation by using a ring of return electrodes around an anode or a cathode to increase or decrease, respectively, the cortical excitability in a much more focal way59. Based on this high focality and the previous tolerability and effects of HD-tDCS study by Borckardt et al.60, the use of HD-tDCS has increased in recent years.
Modeling studies indicate that this electrode configuration generates the highest electric field (EF) intensity beneath the target electrode, with the brain current flow constrained by the radius of the 4 x 1 ring setup and, thus, a larger electric field at the selected target compared to conventional electrode placement60,61,62. The return electrodes contribute to isolating the targeted area, allowing for more focused brain stimulation and producing longer-lasting effects than conventional tDCS63.
In addition, according to some studies, HD-tDCS has longer-lasting effects. Lately, clinical research has been paying attention to this protocol. To our knowledge, only six studies have been conducted with HD-tDCS in neurological diseases, three randomized control trials, two open-label reports, and one case report (refer to the review49).
Although there is no total consensus on the anatomical areas related to hemispatial neglect, there seems to be some agreement on some specific areas. The posterior parietal cortex seems to be the key area of the alteration64,65,66, and within this area, the angular gyrus64,65,67,68,69, the intraparietal sulcus64,69,70, the temporoparietal junction69,71and the supramarginal gyrus65,72,73,74.
Given that the benefit of HD-tDCS compared to conventional tDCS is increasing the precision in the stimulation target and based on the knowledge of a precise location for the presence of hemispatial neglect, we can expect to obtain greater benefits from focal stimulation compared to more general or diffuse stimulation. Meanwhile, the most used configuration in neurology studies is 4 x 1 montage75,76,77,78,79. In our study, we used a 7 x 1 configuration with the aim of increasing the focality of the stimulation even more, being the first study using this montage in clinical rehabilitation of neglect. Therefore, further research in this and other clinical conditions must be conducted to determine the superiority or efficacy of this HD-tDCS montage over other HD montage and conventional tDCS.
Regarding intensity, 2 mA is applied in this protocol, as in most of the studies with tDCS, no matter what montage or configuration is used. It would be interesting to compare the same protocol with lower and higher intensities in further studies to figure out the effect of different applied intensities.
Some useful recommendations regarding safety and technical troubleshooting must be taken into consideration with the current protocol. In every patient, but especially in stroke patients, safety issues should be thoroughly assessed. Although tDCS in stroke patients is safe and well tolerated80, the patients and their families sometimes have doubts about it. Thus, comprehensible information should be handed in advance and discussed with the patient and relatives, ensuring that they understand the procedure and can abandon the protocol whenever they want to.
On the other hand, in this protocol, the exact location of the lesion has been considered and recorded as we are willing to compare the effect of the protocol on hemispatial neglect after cortical lesions (e.g., right middle cerebral artery)69 and after subcortical lesions (e.g., basal ganglia)81. In this context, it is crucial to assess the technique's efficacy in light of the heterogeneity in lesion locations. Specifically, we need to analyze the variations in effectiveness concerning cortical versus subcortical lesions.
Regarding the stroke phase, when applying the stimulation (acute, subacute, or chronic), it is important to know the moment in which the intervention could be most beneficial. In this study, we have used as an inclusion criterion 3 to 12 months since the injury (subacute phase). However, a previous systematic review focused on motor aspects after stroke, and results have shown improvements in chronic phases but not in the acute phase (within the first 3 days from the onset of symptoms)82. Further investigation is necessary to explore the benefits of tDCS on post-stroke cognitive alterations and to identify factors that predict its optimal effectiveness across various stages of recovery.
The current knowledge about HD-tDCS as a therapeutic approach in neurological diseases supports its tolerability and clinical efficacy. Besides, further randomized controlled research is needed to figure out the optimal parameters in each disease and each patient in order to establish the effectiveness of this noninvasive brain stimulation technique in neurological disorders and beyond.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank NeuronUp (www.neuronup.com) for its support and selfless collaboration in this project.
Materials
| Name | Company | Catalog Number | Comments |
| 10 electrode cable | Neuroelectrics | NE017 | |
| Barthel Index | N/A | Mahoney, F. I., Barthel, D. W. Functional evaluation: The Barthel Index. Md State Med J. 14, 61–65 (1965). | |
| Copy of drawings subtest | N/A | https://test-barcelona.com/es/tienda.html | J. Peña Casanova, Programa integrado de exploración neuropsicológica: test Barcelona revisado?: TBR. Barcelona: Masson. |
| Curved Syrenge | Neuroelectrics | NE014 | |
| Electrode Gel | Neuroelectrics | NE016a | |
| Line bisection test | N/A | Schenkenberg, T., Bradford, D. C., Ajax, E. T. Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology. 30 (5) 509–517 (1980). | |
| Mini-mental state examination (MMSE) | N/A | Folstein, M. F., Folstein, S. E., McHugh, P. R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 12 (3) 189–198 (1975). | |
| Neoprene headcap | Neuroelectrics | NE019-M | |
| Saline Solution | Neuroelectrics | NE033 | |
| Satrstim Necbox | Neuroelectrics | NE012 | |
| Starstim tES-EEG System | Neuroelectrics | ||
| Teastboard Cable | Neuroelectrics | NE039 | |
| Testboard Head | Neuroelectrics | NE038 | |
| The Bell Test | N/A | https://strokengine.ca/en/assessments/bells-test/ | L. Gauthier, F. Deahault and Y. Joanette, The Bells Test: A quantitative and qualitative test for visual neglect (Vol. 11). |
| The Catherine Bergego Scale | N/A | Azouvi, P. et al. Behavioral assessment of unilateral neglect: study of the psychometric properties of the Catherine Bergego Scale. Arch Phys Med Rehabil. 84 (1) 51–57 (2003). | |
| The motor-free visual perception test (MVPT) | N/A | https://www.wpspublish.com/mvpt-4-motor-free-visual-perception-test-4 | Colarusso, R. P., Hammill, D.D. The Motor Free Visual Perception Test (MVPT-3). Navato, CA: Academic Therapy Publications (2003). |
| USB Bluetooth Dongle | Neuroelectrics | NE031 | |
| USB charging Cable | Neuroelectrics | NE043 | |
| USB Power Adapter & Power Supply Plug | Neuroelectrics | NE013 & NE013a, NE013b, NE013c | |
| USB Stick with Manuals & NIC SW | Neuroelectrics | NE015 |
References
- Sun, J. -H., Tan, L., Yu, J. -T. Post-stroke cognitive impairment: epidemiology, mechanisms and management. Ann Transl Med. 2 (8), 80(2014).
- Abo, M., et al. comparative study of NEURO versus CIMT in poststroke patients with upper limb hemiparesis: the neuro-verify study. Int J Stroke. 9 (5), 607-612 (2014).
- Mijajlović, M. D., et al. Post-stroke dementia - a comprehensive review. BMC Med. 15 (1), 11(2017).
- Kerkhoff, G., Schenk, T. Rehabilitation of neglect: an update. Neuropsychologia. 50 (6), 1072-1079 (2012).
- Jehkonen, M., et al. Predictors of discharge to home during the first year after right hemisphere stroke. Acta Neurol Scand. 104 (3), 136-141 (2001).
- Buxbaum, L. J., et al. Hemispatial neglect: subtypes, neuroanatomy, and disability. Neurology. 62 (5), 749-756 (2004).
- Nijboer, T. C. W., Kollen, B. J., Kwakkel, G. Time course of visuospatial neglect early after stroke: a longitudinal cohort study. Cortex. 49 (8), 2021-2027 (2013).
- Ringman, J. M., Saver, J. L., Woolson, R. F., Clarke, W. R., Adams, H. P. Frequency, risk factors, anatomy, and course of unilateral neglect in an acute stroke cohort. Neurology. 63 (3), 468-474 (2004).
- Parton, A., Malhotra, P., Husain, M. Hemispatial neglect. J Neurol Neurosurg Psychiatry. 75 (1), 13-21 (2004).
- Stone, S. P., Patel, P., Greenwood, R. J., Halligan, P. W. Measuring visual neglect in acute stroke and predicting its recovery: the visual neglect recovery index. J Neurol Neurosurg Psychiatry. 55 (6), 431(1992).
- Chen, P., Hreha, K., Kong, Y., Barrett, A. M. Impact of spatial neglect on stroke rehabilitation: evidence from the setting of an inpatient rehabilitation facility. Arch Phys Med Rehabil. 96 (8), 1458-1466 (2015).
- Wilkinson, D., Sakel, M., Camp, S. -J., Hammond, L. Patients with hemispatial neglect are more prone to limb spasticity, but this does not prolong their hospital stay. Arch Phys Med Rehabil. 93 (7), 1191-1195 (2012).
- Gammeri, R., Iacono, C., Ricci, R., Salatino, A. Unilateral spatial neglect after stroke: current insights. Neuropsychiatr Dis Treat. 16, 131-152 (2020).
- Muñoz-Marrón, E., Redolar-Ripoll, D., Zulaica-Cardoso, A. New therapeutic approaches in the treatment of neglect: transcranial magnetic stimulation. Rev Neurol. 55 (5), 297-305 (2012).
- Cicerone, K. D., et al. Evidence-based cognitive rehabilitation: systematic review of the literature from 2009 through 2014. Arch Phys Med Rehabil. 100 (8), 1515-1533 (2009).
- Pizzamiglio, L., et al. Cognitive rehabilitation of the hemineglect disorder in chronic patients with unilateral right brain damage. J Clin Exp Neuropsychol. 14 (6), 901-923 (1992).
- Yi, Y., et al. The effect of transcranial direct current stimulation on neglect syndrome in stroke patients. Ann Rehabil Med. 40 (2), 223-229 (2016).
- Fregni, F., Pascual-Leone, A. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Rev Neurol. 3 (7), 383-393 (2007).
- Lefaucheur, J. -P., et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 128 (1), 56-92 (2017).
- González-Rodriguez, B., Serradell-Ribé, N., Viejo-Sobera, R., Romero-Muñoz, J. P., Marron, E. M. Transcranial direct current stimulation in neglect rehabilitation after stroke: a systematic review. J Neurol. 269 (12), 6310-6329 (2022).
- Kinsbourne, M. A model for the mechanism of unilateral neglect of space. Trans Am Neurol Assoc. 95, 143-146 (1970).
- Kinsbourne, M. Hemi-neglect and hemisphere rivalry. Adv Neurol. 18, 41-49 (1977).
- Koch, I. Instruction effects in task switching. Psychon Bull Rev. 15 (2), 448-452 (2008).
- Corbetta, M., Kincade, M. J., Lewis, C., Snyder, A. Z., Sapir, A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci. 8 (11), 1603-1610 (2005).
- Hummel, F. C., Cohen, L. G. Noninvasive brain stimulation: a new strategy to improve neurorehabilitation after stroke. Lancet Neurol. 5 (8), 708-712 (2006).
- Miniussi, C., et al. Efficacy of repetitive transcranial magnetic stimulation/transcranial direct current stimulation in cognitive neurorehabitation. Brain Stimul. 1 (4), 326-336 (2008).
- Bornheim, S., Maquet, P., Croisier, J., Crielaard, J., Kaux, J. Motor cortex transcranial direct current stimulation (tDCS) improves acute stroke visuo-spatial neglect: a series of four case reports. Brain Stimul. 11 (2), 459-461 (2018).
- Sunwoo, H., et al. Effects of dual transcranial direct current stimulation on post-stroke unilateral visuospatial neglect. Neurosci Lett. 554, 94-98 (2013).
- Ladavas, E., et al. A-tDCS on the ipsilesional parietal cortex boosts the effects of prism adaptation treatment in neglect. Restor Neurol Neurosci. 33 (5), 647-662 (2015).
- Turgut, N., Miranda, M., Kastrup, A., Eling, P., Hildebrandt, H. tDCS combined with optokinetic drift reduces egocentric neglect in severely impaired post-acute patients. Neuropsychol Rehabil. 28 (4), 515-526 (2018).
- Smit, M., et al. Transcranial direct current stimulation to the parietal cortex in hemispatial neglect: a feasibility study. Neuropsychologia. 74, 152-161 (2015).
- Brem, A. -K., Unterburger, E., Speight, I., Jancke, L. Treatment of visuospatial neglect with biparietal tDCS and cognitive training: a single-case study. Front Syst Neurosci. 8, 180-180 (2014).
- Cappon, D., Jahanshahi, M., Bisiacchi, P. Value and efficacy of transcranial direct current stimulation in the cognitive rehabilitation: a critical review since 2000. Front Neurosci. 10, 157(2016).
- Fan, J., Li, Y., Yang, Y., Qu, Y., Li, S. Efficacy of noninvasive brain stimulation on unilateral neglect after stroke: a systematic review and meta-analysis. Am J Phys Med Rehabil. 97 (4), 261-269 (2018).
- Kashiwagi, F. T., et al. Noninvasive brain stimulations for unilateral spatial neglect after stroke: a systematic review and meta-analysis of randomized and nonrandomized controlled trials. Neural Plast. , (2018).
- Salazar, A. P. S., et al. Noninvasive brain stimulation improves hemispatial neglect after stroke: a systematic review and meta-analysis. Arch Phys Med Rehabil. 99 (2), 355-366 (2018).
- Zebhauser, P. T., Vernet, M., Unterburger, E., Brem, A. -K. Visuospatial neglect-a theory-informed overview of current and emerging strategies and a systematic review on the therapeutic use of noninvasive brain stimulation. Neuropsychol Rev. 29 (4), 397-420 (2019).
- Bikson, M., et al. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul. 9 (5), 641-661 (2016).
- Antal, A., et al. Low intensity transcranial electric stimulation: safety, ethical, legal regulatory and application guidelines. Clin Neurophysiol. 128 (9), 1774-1809 (2017).
- Sathappan, A. V., Luber, B. M., Lisanby, S. H. The dynamic duo: combining noninvasive brain stimulation with cognitive interventions. Prog Neuropsychopharmacol Biol Psychiatry. 89, 347-360 (2019).
- Draaisma, L. R., Wessel, M. J., Hummel, F. C. Noninvasive brain stimulation to enhance cognitive rehabilitation after stroke. Neurosci Lett. 719, 133678(2020).
- David, M. C. M. M., Moraes, A. A., de Costa, M. L., da Franco, C. I. F. Transcranial direct current stimulation in the modulation of neuropathic pain: a systematic review. Neurol Res. 40 (7), 557-565 (2018).
- Dondé, C., et al. Transcranial direct-current stimulation (tDCS) for bipolar depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 78, 123-131 (2017).
- Gowda, S. M., et al. Efficacy of pre-supplementary motor area transcranial direct current stimulation for treatment resistant obsessive compulsive disorder: a randomized, double blinded, sham controlled trial. Brain Stimul. 12 (4), 922-929 (2019).
- Kang, N., Summers, J. J., Cauraugh, J. H. Transcranial direct current stimulation facilitates motor learning post-stroke: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 87 (4), 345-355 (2016).
- Narayanaswamy, J. C., et al. Successful application of add-on transcranial direct current stimulation (tDCS) for treatment of SSRI resistant OCD. Brain Stimul. 8 (3), 655-657 (2015).
- Osoegawa, C., et al. Noninvasive brain stimulation for negative symptoms in schizophrenia: an updated systematic review and meta-analysis. Schizophr Res. 197, 34-44 (2018).
- Vacas,, et al. Noninvasive brain stimulation for behavioural and psychological symptoms of dementia: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 34 (9), 1336-1345 (2019).
- Parlikar, R., et al. High definition transcranial direct current stimulation (HD-tDCS): a systematic review on the treatment of neuropsychiatric disorders. Asian J Psychiatry. 56, 102542(2021).
- Fried, P. J., et al. Training in the practice of noninvasive brain stimulation: recommendations from an IFCN committee. Clin Neurophysiol. 132 (3), 819-837 (2021).
- Cappa, S. F., et al. EFNS guidelines on cognitive rehabilitation: report of an EFNS task force. Eur J Neurol. 12 (9), 665-680 (2005).
- Sturm, W., Willmes, K., Orgass, B., Hartje, W. Do specific attention deficits need specific training. Neuropsychol Rehabil. 7 (2), 81-103 (1997).
- Verveer, I., Remmerswaal, D., Van der Veen, F. M., Franken, I. H. A. Long-term tDCS effects on neurophysiological measures of cognitive control in tobacco smokers. Biol Psychol. 156, 107962(2020).
- Katz, B., et al. Individual differences and long-term consequences of tDCS-augmented cognitive training. J Cogn Neurosci. 29 (9), 1498-1508 (2017).
- Gu, J., et al. The effect and mechanism of transcranial direct current stimulation on episodic memory in patients with mild cognitive impairment. Front Neurosci. 16, 811403(2022).
- Zhou, Y., et al. Efficacy and safety of transcranial direct current stimulation (tDCS) on cognitive function in chronic schizophrenia with tardive dyskinesia (TD): a randomized, double-blind, sham-controlled, clinical trial. BMC Psychiatry. 23 (1), 623(2023).
- Au, J., et al. Enhancing working memory training with transcranial direct current stimulation. J Cogn Neurosci. 28 (9), 1419-1432 (2016).
- Stagg, C. J., Antal, A., Nitsche, M. A. Physiology of transcranial direct current stimulation. J ECT. 34 (3), 144-152 (2018).
- Da Silva Machado, D. G., et al. Acute effect of high-definition and conventional tDCS on exercise performance and psychophysiological responses in endurance athletes: a randomized controlled trial. Sci Rep. 11, 13911(2021).
- Borckardt, J. J., et al. A pilot study of the tolerability and effects of high-definition transcranial direct current stimulation (HD-tDCS) on pain perception. J Pain. 13 (2), 112-120 (2012).
- Villamar, M. F., et al. Technique and considerations in the use of 4x1 ring high-definition transcranial direct current stimulation (HD-tDCS). J Vis Exp. (77), e50309(2013).
- Effects of electrode configurations and injected current intensity on the electrical field of transcranial direct current stimulation: a simulation study. Mackenbach, C., Tian, R., Yang, Y. Annu Int Conf IEEE Eng Med Biol Soc, , 3517-3520 (2020).
- Bikson, M., Datta, A., Rahman, A., Scaturro, J. Electrode montages for tDCS and weak transcranial electrical stimulation: role of "return" electrode's position and size. Clin Neurophysiol. 121 (12), 1976-1978 (2010).
- Chambers, C. D., Stokes, M. G., Mattingley, J. B. Modality-specific control of strategic spatial attention in parietal cortex. Neuron. 44 (6), 925-930 (2004).
- Chambers, C. D., Payne, J. M., Mattingley, J. B. Parietal disruption impairs reflexive spatial attention within and between sensory modalities. Neuropsychologia. 45 (8), 1715-1724 (2007).
- Chambers, C. D., Mattingley, J. B. Neurodisruption of selective attention: insights and implications. Trends Cogn Sci. 9 (11), 542-550 (2005).
- Rushworth, M. F., Ellison, A., Walsh, V. Complementary localization and lateralization of orienting and motor attention. Nat Neurosci. 4 (6), 656-661 (2001).
- Hillis, A. E., et al. Anatomy of spatial attention: insights from perfusion imaging and hemispatial neglect in acute stroke. J Neurosci. 25 (12), 3161-3167 (2005).
- Mort, D. J., et al. The anatomy of visual neglect. Brain. 126 (9), 1986-1997 (2003).
- Mannan, S. K., et al. Revisiting previously searched locations in visual neglect: role of right parietal and frontal lesions in misjudging old locations as new. J Cogn Neurosci. 17 (2), 340-354 (2005).
- Vallar, G. Extrapersonal visual unilateral spatial neglect and its neuroanatomy. Neuroimage. 14 (1 Pt 2), S52-S58 (2001).
- Oliveri, M., Vallar, G. Parietal versus temporal lobe components in spatial cognition: setting the mid-point of a horizontal line. J Neuropsychol. 3 (2), 201-211 (2009).
- Committeri, G., et al. Neural bases of personal and extrapersonal neglect in humans. Brain. 130 (Pt 2), 431-441 (2007).
- Doricchi, F., Tomaiuolo, F. The anatomy of neglect without hemianopia: a key role for parietal-frontal disconnection. Neuroreport. 14 (17), 2239-2243 (2003).
- Fiori, V., Nitsche, M. A., Cucuzza, G., Caltagirone, C., Marangolo, P. High-definition transcranial direct current stimulation improves verb recovery in aphasic patients depending on current intensity. Neuroscience. 406, 159-166 (2019).
- Karvigh, S. A., Motamedi, M., Arzani, M., Roshan, J. H. N. HD-tDCS in refractory lateral frontal lobe epilepsy patients. Seizure. 47, 74-80 (2017).
- Meiron, O., et al. Antiepileptic effects of a novel noninvasive neuromodulation treatment in a subject with early-onset epileptic encephalopathy: case report with 20 sessions of HD-tDCS intervention. Front Neurosci. 13, (2019).
- Reckow, J., et al. Tolerability and blinding of 4x1 high-definition transcranial direct current stimulation (HD-tDCS) at two and three milliamps. Brain Stimul. 11 (5), 991-997 (2018).
- Motes, M. A., et al. High-definition transcranial direct current stimulation to improve verbal retrieval deficits in chronic traumatic brain injury. J Neurotrauma. 37 (1), 170-177 (2020).
- Russo, C., Souza Carneiro, M. I., Bolognini, N., Fregni, F. Safety review of transcranial direct current stimulation in stroke. Neuromodulation. 20 (3), 215-222 (2017).
- Hochstenbach, J., Van Spaendonck, K. P., Cools, A. R., Horstink, M. W., Mulder, T. Cognitive deficits following stroke in the basal ganglia. Clin Rehabil. 12 (6), 514-520 (1998).
- Marquez, J., Van Vliet, P., McElduff, P., Lagopoulos, J., Parsons, M. Transcranial direct current stimulation (tDCS): does it have merit in stroke rehabilitation? A systematic review. Int J Stroke. 10 (3), 306-316 (2015).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved