Method Article
Separation and Differential Characterization of Gut Microbial Extracellular Vesicles in Salt-Sensitive Rats under High-Salt Diet Conditions
* These authors contributed equally
In This Article
Summary
The protocol describes the isolation of gut microbial EVs from salt-sensitive rats fed HSD using density gradient centrifugation. EVs were characterized by nanoparticle tracking, TEM, LPS/BCA assays, and 16S rRNA sequencing to analyze the size, morphology, composition, and microbiota origin.
Abstract
High salt intake is a major risk factor for hypertension, and its underlying mechanism may be closely linked to extracellular vesicles (EVs) secreted by gut microbiota. These EVs, produced by gut microbiota, carry various bioactive components that may play a crucial role in the development of hypertension induced by a high-salt diet (HSD). To investigate this mechanism, we developed an efficient extraction method based on density gradient centrifugation to isolate EVs from the gut microbiota of salt-sensitive rats fed an HSD. Through particle size analysis, transmission electron microscopy (TEM), and lipopolysaccharide (LPS) detection, we identified the gradient distribution of gut microbiota EVs and achieved precise extraction. Furthermore, 16S rRNA gene sequencing was employed to analyze the origin and compositional differences of EVs between the normal and HSD groups, revealing the impact of high salt intake on the genetic characteristics of gut microbiota EVs. This study provides valuable tools and scientific insights into the gut microbiota mechanisms underlying salt-induced hypertension and offers new perspectives for the prevention and treatment of related diseases.
Introduction
The gut microbiota, also known as gut microbiota microflora or gut microecology, is a complex of tens of thousands of microorganisms located in the biological gastrointestinal tract and plays a crucial role in the maintenance of human health1. In recent years, with further research, it has been found that the gut microbiota can produce extracellular vesicles (EVs)2. EVs are small vesicles released by cells, which carry various molecules in the cell, such as proteins, nucleic acids, and lipids3,4. They can interact with other microbes5, intestinal epithelial cells, and even distant tissues and organs6, thus affecting the health of the human body7,8. There is a tight link between the EVs produced by these gut microbiota and diet9.
EVs produced by gut microbiota may be significant agents through which a high-salt diet (HSD) affects body health. HSD not only directly disrupts the balance of gut microbiota10, leading to a significant reduction in the number of beneficial bacteria (such as Lactobacillus)11, but also promotes the proliferation of harmful bacteria (such as Bacteroides, etc.)12. This imbalance reduces the intestinal barrier function and increases the risk of intestinal inflammation. In addition, an HSD also further affects the acid-base balance and nutrient absorption in the intestine by changing the metabolic activities13 of the gut microbiota, such as reducing the production of short-chain fatty acids14,15 with multiple physiological functions.
These changes not only impact intestinal health but may also indirectly regulate the production and release of EVs and alter the EV's composition and function. The high salt environment may affect the normal physiological functions of intestinal cells, including the release and transport of EVs, thereby disturbing the role of EVs in intercellular information transmission and immune regulation. At the same time, intestinal inflammation may promote a variety of EVs with special functions16 and spread to the whole body through the intestinal-organ axis and other ways17,18, which is closely related to the occurrence and development of hypertension19,20, cardiovascular21 and cerebrovascular diseases22,23, obesity24,25, diabetes26 and other chronic diseases.
Therefore, the overall goal of this study was to develop an efficient and reliable method to extract EVs from the gut microbiota of salt-sensitive rats fed an HSD and to systematically study their physical properties, composition, and functions. Due to the characteristics of the significant increase in blood pressure after a high-salt diet, salt-sensitive rats were selected and revealed the effect of HSD on gut microbiota EV by constructing an efficient extraction method. The method was based on density gradient centrifugation and combined various dynamic identification techniques such as particle size detection, LPS/BCA measurement, transmission electron microscopy, and proteomic analysis. The protocol aims to reveal the effects of HSD on gut microbiota EV and its mechanisms in cardiovascular disease. With its high efficiency, reproducibility, and broad applicability, this approach not only provides an important tool for exploring the mechanism of gut microbiota EVs in salt-induced hypertension but also lays the theoretical foundation for developing disease intervention strategies based on EVs. Through this study, we hope to open up new avenues for the prevention and treatment of cardiovascular diseases, such as hypertension27,28.
Protocol
This animal experimental study complies with the relevant ethical guidelines and international standards. Studies involving animals were approved by the Laboratory Animal Welfare and Ethics Committee of Chengdu University of Chinese Medicine (institution: Chengdu University of Chinese Medicine; protocol number: 2018-21).
1. Animal preparation and diet regime
- Acclimatize 6-week-old male Dahl salt-sensitive rats, body weight 180-200 g, by rearing at 50% ± 10% humidity; 12 h / 12 h light cycle; 20.0 ± 2.0 °C for 1 week under specific pathogen-free conditions.
- Subsequently, divide the rats equally into two groups (n = 6). Provide each group a different diet that lasting for 6 weeks: a normal salt diet (NSD) with 0.5% sodium chloride and an HSD with 8% sodium chloride, which is commonly used in published studies29,30,31. The mice had unlimited access to food and water throughout the study.
2. Monitoring of blood pressure
NOTE: Tail cuff plethysmography was used as a non-invasive method for blood pressure measurement, and volumetric pressure recording (VPR) was used when blood pressure was measured from tail blood volume.
- Place rats individually in small cages with sawdust, heated for 10 min at approximately 37 °C and place in a custom rodent restraint with an adjustable nose and door for animal entry. Place rats on a heating pad in a prone position to keep their body temperature at 37 °C.
- Connect the VPR sensor to the bottom of the tail and set at least 10 adaptation cycles to stabilize the BP28.
- Fill the inflatable cuff on the tail root and place the plethysmographic sensor (or photoelectric sensor) below. Slowly inflate to above systolic pressure (usually 200 mmHg) and then slowly release. The instrument automatically detects the pressure value of pulse wave disappearance (systolic blood pressure) and recovery (diastolic blood pressure). Measure 3-5 consecutive times and take the average value.
- Train all rats to accommodate the restraint procedure and perform this study in a quiet laboratory around 9 am to minimize the effects of circadian variation.
- Measure systolic blood pressure (SBP) and diastolic blood pressure (DBP) every other day before sacrifice. Estimate mean arterial pressure (MAP) from SBP and DBP
MAP = (SBP + 2 DBP)/3
3. Extraction of EVs
- Sample collection
- Dip a sterile cotton swab in 75% alcohol and use it to stimulate the rat's anus. The stimulation was intended to promote intestinal peristalsis and relax the anal sphincter, thereby promoting defecation. After the feces are expelled, collect them using sterile forceps into appropriately sized sterile containers and immediately store them at -80 °C.
- Sample preparation
- Using a spoon, transfer 5 g of feces into a pre-weighed 50 mL centrifuge tube. Add 50 mL of pre-heated 37 °C endotoxin-free PBS (maximum sample concentration 10%) to the tube and rotate for 30 min. Cool the high-speed centrifuge to 4 °C.
- Centrifuge at 8,000 x g for 15 min after placing samples symmetrically to allow the centrifuge to equilibrate. After centrifugation, aspirate the supernatant and transfer it to a new sterile centrifuge tube.
- Centrifuge again at 8,000 x g for 15 min. Aspirate the supernatant and use it for further analysis.
- Crude extract preparation
- Use a sterile 0.22 µm filter unit. Place the filter unit on ice and hold the vacuum pump firmly. Transfer the supernatant obtained to the top of the filter. Turn on the vacuum pump to collect the filtered samples.
- Transfer the filtrate to a centrifugal filter (10 kDa, 15 mL), centrifuge at 4 °C, 3,000 x g for 30 min, and concentrate the sample to at least 1400 µL.
- Collect the concentrated sample and, if necessary, dilute it to 1400 µL using pre-cooled endotoxin-free PBS. Keep the sample on ice immediately after dilution.
NOTE: The crude extract can be used immediately or stored at -80 °C for several months.
- Separation of EVs
- Prepare the gradient buffer A and gradient buffer B as described below. Prepare the buffer one day in advance, as it takes several hours for the compound to dissolve completely. The prepared buffer can be stored at 4 °C for 6 months.
- Preparation of gradient buffer A: Dissolve 0.25 M sucrose, 6 mM EDTA, and 60 mM Tris in 800 mL of endotoxin-free water by magnetic mixer stirring, with the pH adjusted to 7.4 using hydrochloric acid. Dilute to 1 L with endotoxin-free water. Use a 0.22 µm bottle-top filter to filter the buffer.
- Preparation of gradient buffer B: Dissolve 0.25 M sucrose, 1 mM EDTA, and 10 mM Tris in 800 mL of endotoxin-free water by magnetic mixer stirring, with pH adjusted to 7.4 with hydrochloric acid. Diluted to 1 L with endotoxin-free water. Use a 0.22 µm bottle top filter to filter the buffer.
- Mix 1 volume of gradient buffer A and 5 volumes of density gradient medium (the medium is 60% iodixanol solution) to prepare the working solution.
- Preparation of iodixanol solution (10%, 20%, 40%): To prepare a 10% iodixanol solution, mix 1 unit working solution and 4 units buffer B; for 20% iodixanol solution, mix 2 unit working solution and 3 units buffer B; for 40% iodixanol solution, mix 4 units working solution and 1 unit buffer B. Prepare each solution fresh and store on ice.
NOTE: Fresh working solutions should be prepared for each experiment, and an additional 10% volume of solutions can be prepared to account for the experimental error. - Mix the solution prepared in step 3.3.3 with 7 mL of density gradient medium to make a 50% iodixanol solution.
- To prepare a density gradient, add trypan blue solution to 40% solution and 10% (wt/vol) iodixanol solution to provide a clear outline between the distinct layers.
- Transfer 8 mL of 50% iodixanol solution to the bottom of the thin-wall polypropylene centrifuge tube. Tilt the tube to 70° and transfer 8 mL of 40% iodixanol solution to the liquid surface. Add 8 mL of 20% iodixanol solution, then 7 mL of 10% iodixanol solution, and finally 2 mL of endotoxin-free PBS.
NOTE: Preparing a density gradient requires practice. The quality of the density gradient has a great impact on the experimental results. Do not place the tube upright between different steps in the addition process, as this negatively impacts the quality of the density gradient. - Precool the centrifuge in advance and place the prepared density gradient into the ultracentrifuge. Input parameters to start, parameter values: 100,000 x g, 20 h, 10 °C.
- For manual gradient density separation, slowly draw 2 mL of the solution from the center of the surface of the system containing a total of 34 mL of liquid. Each 2mL is a gradient, thus dividing the density gradient solution into 17 gradients.
- Transfer the density fractions to a sterile sample tube using a pipette and immediately place them on ice. Clamp the centrifuge tube between the thumb and index finger to ensure that it remains upright.
- Prepare the gradient buffer A and gradient buffer B as described below. Prepare the buffer one day in advance, as it takes several hours for the compound to dissolve completely. The prepared buffer can be stored at 4 °C for 6 months.
- Recovery of EVs
- Using a fully automated EVs extraction system that applies negative pressure oscillation through a double coupling ultrasonic oscillation system on the ultrafiltration chip, remove nucleic acid and protein impurities in the sample through the nanopore and intercept EVs, leading to enrichment and purification of EVs.
- Remove the filtrate in step 3.4.7 and add it to the ultrafiltration chip.
- Operate the instrument according to the instrument instructions and collect the filtered filtrate, namely the exosome-enriched solution. The main process is shown in Figure 1.
- Using a fully automated EVs extraction system that applies negative pressure oscillation through a double coupling ultrasonic oscillation system on the ultrafiltration chip, remove nucleic acid and protein impurities in the sample through the nanopore and intercept EVs, leading to enrichment and purification of EVs.
4. EVs identification
- Particle size and concentration detection
- Evaluate the size distribution and zeta potential using a resistive pulse sensing (RPS) -based nanocoulometer counter. Select nanopore chips with a measurement range of 60-200 nm for this experiment. Further, evaluate the particle size distribution and zeta potential of the EVs using a nanolibrary counter.
- Take an appropriate amount of EVs solution and dilute it in (1/10, 1/100, 1/1000, 1/10000).
- Different dilutions of EVs solutions were placed on the instrument for testing.
- The dilution multiple with better stability was selected and tested again to obtain more accurate data.
- Evaluate the size distribution and zeta potential using a resistive pulse sensing (RPS) -based nanocoulometer counter. Select nanopore chips with a measurement range of 60-200 nm for this experiment. Further, evaluate the particle size distribution and zeta potential of the EVs using a nanolibrary counter.
- LPS and BCA assay
- BCA assay: Lyse EVs using RIPA buffer and then separate the proteins. Determine the protein content of the EVs using the BCA protein concentration determination kit.
- LPS assay: Using the endotoxin detection kit, add samples, an endotoxin detector, and chromogenic according to the kit steps. Measure the absorbance at 545 nm and calculate the LPS expression of external vesicles according to the standard curve.
- TEM evaluation: For negative staining and TEM analysis, apply samples to a luminescent copper grille coated with continuous carbon film and stain with 0.75% uranyl formate. For cryo-EM, absorb the EVs into a 300-mesh EM carbon grid with a hydrophilic surface and freeze in liquid nitrogen. Observe and analyze the grids using the Cryo-TEM and record the images at 22,000 magnifications32.
- Characterization of proteins: Lyse EVs using RIPA buffer and then separate the proteins. Determine protein concentration using the BCA protein assay kit, separate equal amounts of protein from each sample by SDS-PAGE, and stain with Coomassie blue dye solution after electrophoresis to visualize the protein profiles33,34.
- S sequencing and analysis: Extract genomic DNA from the fecal samples using a fecal DNA extraction kit. Assess the integrity and concentration of DNA by agarose gel electrophoresis and a spectrophotometer. Amplify the V3 - V4 region of the 16S rRNA gene with universal primers, purify the PCR products, quantify them, and sequence them on the Illumina platform. Perform data analysis using the QIIME2 pipeline35.
- Classify amplicon sequence variants (ASVs) using DADA2 and match against the Silva database using the VSEARCH algorithm. Use α-Diversity analysis to assess species richness and diversity within the samples, mainly using indices such as the Shannon index and the Chao1 index. β-Diversity analysis compares the microbial community composition between samples and is usually visualized by principal coordinate analysis (PCoA). Use differential analysis methods, such as linear discriminant analysis effect size (LEfSe) and volcano plot analysis, to identify microbial taxa with significantly different conditions or between groups.
Results
EVs concentrations were determined in different fractions (Figure 2A). The experimental results showed that the concentration of the EVs exhibited a typical normal distribution pattern in a series of density gradient solutions (Figure 2B). Specifically, in fraction 9, the concentration of EVs reached its highest point (3.85 x 109), suggesting that the main distribution fraction of EVs may be 936.
For the determination of protein content, BCA kits were used to assess the protein content in different fractions (Figure 2C). In this method, the protein content in fraction 9 was 0.417 µg/µL, which further demonstrates the distribution of the EVs. Since LPS is a unique component of Gram-negative bacteria37,38, endotoxin detection kits were also used to determine the expression of LPS (Figure 2D) in order to assess the distribution of EVs in different fractions. The experimental results showed that in fractions 9 and 10, LPS expression was significantly higher than the other fractions (absorbance for fraction 9 = 0.8086, for fraction 10 = 0.8515), and its distribution showed a normal distribution, which proved that the EVs are mainly distributed in fraction 9. Relatively greater amounts of protein in fraction 9 were also observed in SDS-PAGE gel electrophoresis of EVs isolated from different fractions (Figure 2E).
This study examined EVs using transmission electron microscopy (TEM; Figure 2F). Through high-resolution imaging by TEM, it was able to clearly visualize the morphological structure of EVs, featuring circular membrane-like structures, which is crucial for understanding the biology of EVs.
Blood pressure changes were determined in the NSD and HSD groups after 2 months of rearing in the NSD and HSD of the rats. We can see that the SBP (Figure 3A), DBP (Figure 3B), and MBP (Figure 3C) were significantly increased in the HSD group, indicating that the hypertension model of this study was successfully constructed.
In this study, the concentration of EVs was detected in different groups (Figure 3D), and the results showed that the concentration of EVs in the HSD group was significantly higher than that of rats in the NSD group. This suggests that HSD affects the level of EVs, potentially due to changes in the composition of the gut microbiota from which the EVs originate.
The particle size of the EVs was then measured in this study. The measurement results indicate that the particle size of the EVs is predominantly around 60 nm (Figure 3E), and the particle size of the HSD group is slightly lower than that of the NSD group. The measured data provides important information for assessing the size distribution and homogeneity of the EVs, facilitating the subsequent experimental design and application development.
Then, in this study, the amount of LPS expression of EVs in the HSD group and NSD group was examined (Figure 3F). The results showed that the expression of LPS by EVs in the HSD group was also significantly higher than that in the NSD group. This may be due to the increase in Gram-negative bacteria in the exovesicle-derived gut microbiota or by the higher concentration of EVs in the HSD group than in the NSD group.
To further support the differences between EVs in the HSD and NSD groups, this study investigated the derived gut microbiota and conducted 16S rRNA sequencing of EVs samples from the NSD and HSD groups to further clarify the effect of HSD on EVs in the gut microbiota in mice.
α-Diversity analysis showed that the diversity of the gut microbiota was significantly reduced in the HSD group, with both the Shannon and ACE indices decreasing (Figure 4A). β -Diversity analysis, based on PCoA (Figure 4B), distinguished the microbial phenotypes between the groups at the ASV level. The high salt intervention partially reversed the phenotypic changes in the gut microflora and found significant differences in the composition of the external vesicle-derived gut microbiota between the groups (p = 0.001).
After filtering out low-abundance bacteria and standardizing the data, taxonomic annotation identified different ranges of microbial communities in the samples. The exovesicle-derived gut microbiota mainly consisted mainly of Proteobacteria (93.19%), Firmicutes (4.57%), and Bacteroidota (1.19%; Figure 4C). The HSD group significantly increased the abundance of Firmicutes external vesicles (Figure 4D). At the genus level, the results showed that genera such as Nevskia and Acinetobacter were more abundant in the NSD group, while Delftia, Burkholderia_Ca, and Clostridium_sen were more abundant in the HSD group (Figure 4E). In addition, this study also used heat maps to show the differences in the gut microbiota EVs between the HSD group and the NSD group (Figure 4F). After the high-salt intervention, the abundance of some bacteria, Delftia and Burkholderia_Ca, increased significantly, and Nevskia and Acinetobacter decreased significantly (Figure 4G). In conclusion, the high salt intervention significantly changed the difference in the production of intestinal microflora-derived EVs, mainly because it changed the distribution of their parental bacteria, and the main characteristics were decreases in diversity, changes in the equilibrium structure, and changes in the abundance of different bacteria.
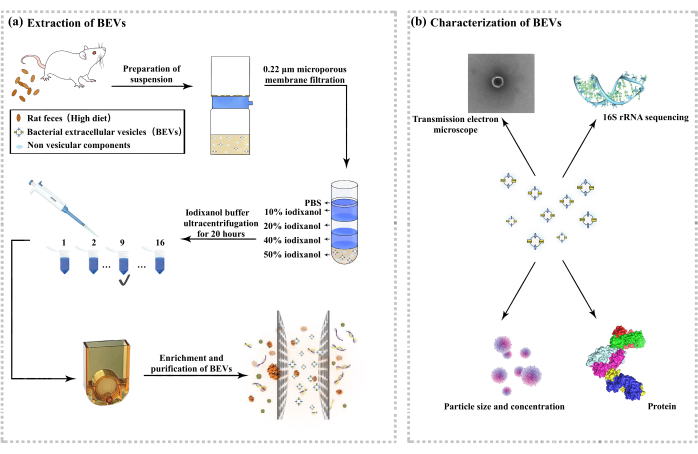
Figure 1: Extraction and characterization of gut microbial-derived outer vesicles. (A) Flow chart of outer vesicle extraction. (B) Characterization of the outer vesicles. Please click here to view a larger version of this figure.
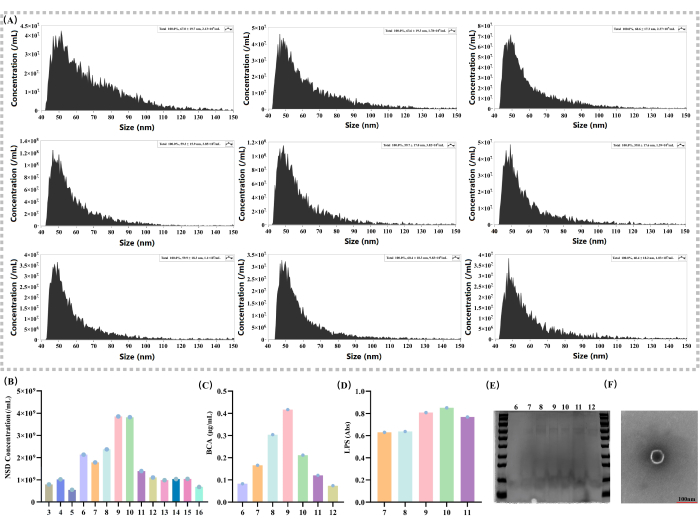
Figure 2: Basic characterization of the EVs and the analysis of the local density gradient. (A) Extra-vesicle concentration and particle size measurements with different density gradients (nm; particles/mL). (B) Comparison of solution external vesicle concentrations by density gradient 3 - 16 (particles/mL). (C) Solution protein content determination on density gradient 6-12 by the BCA kit (µg/mL). (D) Solution LPS expression measurement by density gradient 7-11 with an endotoxin detection kit (Abs). (E) Solutions with a density gradient of 6 - 12 were subjected to SDS-PAGE gel electrophoresis and stained with Coomassie brilliant blue. (F) TEM: The scale bar is 100 nm; individual vesicles are shown in the image. Please click here to view a larger version of this figure.
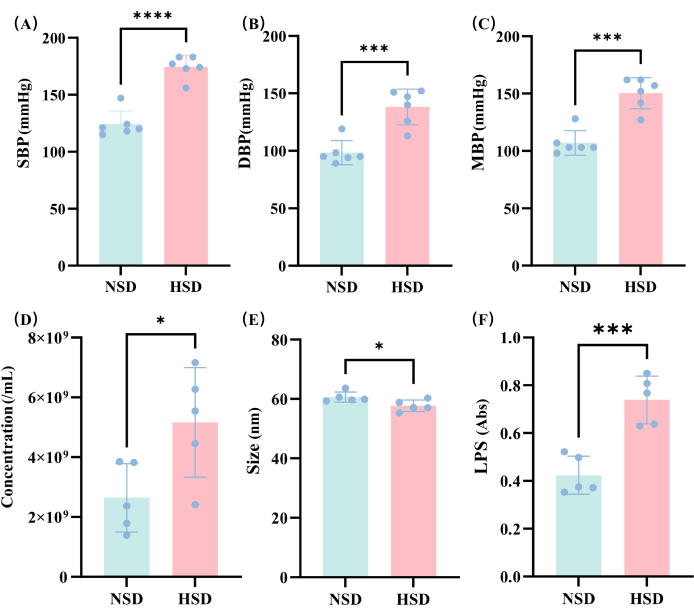
Figure 3: Analysis of the changes in blood pressure and EVs under different diets. (A) SBP; (B) DBP; (C) MBP; (D) Comparison of external vesicle concentrations in the HSD and NSD groups; (E) Comparison of outer vesicle size in the HSD and NSD groups. (F) LPS in the NSD and HSD samples determined by spectrophotometry. *p < 0.05, ** p < 0.01, *** p < 0.001, and NS means no significance, t-test. Please click here to view a larger version of this figure.

Figure 4: Analysis of the 16s rRNA-test under the different diets. (A) The α-diversity analysis; (B) Principal coordinates analysis; (C) the abundance of gut microbiota at the phyla level; (D) Changes in phylum-level bacteria; (E) LEfSe result; (F) the cluster heatmap analysis based on differential bacterial genera; (G) Changes in the genus-level bacteria. *p < 0.05, ** p < 0.01, *** p < 0.001, and NS means no significance, t-test. Please click here to view a larger version of this figure.
Discussion
In this study, we focused on the gut microbiota EVs in salt-sensitive rats on an HSD and achieved a series of key achievements. First, an efficient extraction method based on density gradient centrifugation was successfully constructed to isolate EVs from salt-sensitive rat gut microbiota on HSD, and most non-EV components were isolated through a meticulous, standardized animal experimental manipulation and sample processing process. The EVs extraction method of density gradient centrifugation is highly efficient and reproducible, better than the conventional ultracentrifugation method, and can better retain the integrity and functionality of EVs, ensuring the sample quality and study feasibility.
Secondly, EVs were comprehensively identified through various dynamic technologies: particle size and concentration detection indicate that EVs have a specific size distribution; LPS and BCA measurements quantify the protein content and LPS expression of EVs; TEM clearly shows the morphological structure of EVs; and protein characteristics define the protein spectrum. These identification results comprehensively reveal the physical and biochemical properties of EVs, providing reliable data support for subsequent studies.
In addition, 16S rRNA gene sequencing technology using in-depth analysis of the differences between the origin and composition of normal and HSD EVs, and α-diversity analysis and β-diversity analysis showed that high salt intake significantly affects the genetic characteristics of gut microbial EVs, including microbial community structure, species richness, and diversity, to more comprehensively resolve the effects of high salt diet on gut microbiota EVs, provides multiple levels of evidence for mechanistic studies.
Although this method has some advantages over the conventional overspeed centrifugation method in terms of exosome recovery and integrity, there are still some limitations, such as high sample demand and difficulty in distinguishing the host from the microflora source. However, compared with existing methods, density gradient centrifugation has unique advantages in maintaining the functional integrity of exosomes, which provides reliable technical support for further investigation into the mechanism by which a high-salt diet affects host blood pressure through intestinal flora. Moreover, for the unclear gradient stratification encountered during the experiment, we also improved by extending the centrifugation time or replacing the higher-performance rotor.
In the future, we can further explore the mechanism of gut microbiota EVs in hypertension induced by a high salt diet, especially its interaction with the immune system, such as pro-inflammatory T cells39,40; or use plant-derived products to intervene in gut microbiota EVs41,42, further intervene in diseases and explore their role in regulating host metabolism and immune response.
In conclusion, this study not only provides an important tool to explore the mechanism of salt-induced hypertension of gut microbiota, through the salt-sensitive rat model, deepen the understanding of the relationship between HSD, gut microbiota and EVs, and also opened up a new way for the prevention and treatment of cardiovascular diseases such as hypertension, provides a unique perspective for the study of diet-microbe-host interaction.
Disclosures
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82205240), the Natural Science Foundation of Sichuan Province (2025ZNSFSC1836), and the Clinical Basic Project of Sichuan Provincial Orthopedic Hospital (PY202414).
Materials
| Name | Company | Catalog Number | Comments |
| Essential Supplies | |||
| Centrifugal Filter (10nkDa 2 mL) | Millipore | UFC903096 | |
| Centrifuge Tude(50 mL) | BKMAN | 20220404 | |
| Centrifuge Tudes | BECKMAN COULTER | Z30815SCA | |
| Vacuum Filtration System | Biosharp | 24902581 | |
| Reagents | |||
| Chromogenic LAL Endotoxin Assay Kit | Beyotime | 022124240705 | |
| Coomassie Blue Fast Staining Solution | Beyotime | Z972241010 | |
| EDTA | Damas-beta | P3117308 | |
| Enhanced BCA Protein Assay Kit | Beyotime | A006241112 | |
| Ethanol | KESH | ||
| HCl | |||
| OptiPrep (60% wt/vol, iodixanol) | Serumwerk | 00124 | |
| PBS | Labshark | 130114005 | |
| phosphotungstic acid | RUIXIN | ||
| Sucrose | Damas-beta | P1917057 | |
| Tris (VWR) | Damas-beta | P3061764 | |
| Trypan blue staining solution (0.4%) | Beyotime | BD07242904 | |
| Equipment | |||
| Absorbance Microplate Reader | SpectraMax | ABP01690 | |
| Biomicroscope | Motic | BA210Digital | |
| Desk centrifuge | Cence | CHT210R | |
| Desktop high-speed micro centrifuge | DLAB | D3024 | |
| Fixed Angle Aluminum Rotor + 500 mL Centrifugal Cup | Cence | ||
| High precision electronic balance | SKR | BN-200 | |
| Laminar flow cabinet | Nantong Hunan Scientific Instrument Co., Ltd. | SW-CJ-2FDS | |
| SW 32.1 Ti Swing bucket turn+ SW 32.1 Ti Rotor bucket | BECKMAN COULTER | ||
| Transmission electron microscope | JEOL | JEM-1400FLASH | |
| Tube rotator | |||
| Ultracentrifuge | BECKMAN COULTER | Optima XE-100 | |
| Ultra-pure water system | ULPHW | UPR-II-15TNZ | |
| Water-Cieculation Multifunction Vacuum Pump | Qiang Qiang | SHZ-D(III) |
References
- Thursby, E., Juge, N. Introduction to the human gut microbiota. Biochem J. 474 (11), 1823-1836 (2017).
- Aschtgen, M. S., et al. Rotation of Vibrio fischeri Flagella Produces Outer Membrane Vesicles That Induce Host Development. J Bacteriol. 198 (16), 2156-2165 (2016).
- Gurung, S., Perocheau, D., Touramanidou, L., Baruteau, J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Comm Signal. 19 (1), 47(2021).
- Avila-Calderón, E. D., et al. Outer Membrane Vesicles of Gram-Negative Bacteria: An Outlook on Biogenesis. Front Microbiol. 12, 557902(2021).
- Melo-Marques, I., Cardoso, S. M., Empadinhas, N. Bacterial extracellular vesicles at the interface of gut microbiota and immunity. Gut Microbes. 16 (1), 2396494(2024).
- Kim, N. Y., et al. Effect of gut microbiota-derived metabolites and extracellular vesicles on neurodegenerative disease in a gut-brain axis chip. Nano Converg. 11 (1), 7(2024).
- Ahmadi Badi, S., et al. Microbiota-Derived Extracellular Vesicles as New Systemic Regulators. Front Microbiol. 8, 1610(2017).
- Barathan, M., Ng, S. L., Lokanathan, Y., Ng, M. H., Law, J. X. The Profound Influence of Gut Microbiome and Extracellular Vesicles on Animal Health and Disease. Int J Mol Sci. 25 (7), 4024(2024).
- Maukonen, J., Saarela, M. Human gut microbiota: does diet matter. Proc Nutri Soc. 74 (1), 23-36 (2015).
- Bier, A., et al. A High Salt Diet Modulates the Gut Microbiota and Short Chain Fatty Acids Production in a Salt-Sensitive Hypertension Rat Model. Nutrients. 10 (9), 1154(2018).
- Aghamohammad, S., et al. Anti-inflammatory and immunomodulatory effects of Lactobacillus spp. as a preservative and therapeutic agent for IBD control. Immun Inflamm Dis. 10 (6), e635(2022).
- Dong, Z., et al. The Effects of High-Salt Gastric Intake on the Composition of the Intestinal Microbiota in Wistar Rats. Med Sci Monit. 26, e922160(2020).
- Yan, X., et al. Intestinal Flora Modulates Blood Pressure by Regulating the Synthesis of Intestinal-Derived Corticosterone in High Salt-Induced Hypertension. Circ Res. 126 (7), 839-853 (2020).
- Wang, X., Lang, F., Liu, D. High-Salt Diet and Intestinal Microbiota: Influence on Cardiovascular Disease and Inflammatory Bowel Disease. Biology. 13 (9), 674(2024).
- Qi, L., et al. Microbiome-metabolome analysis insight into the effects of high-salt diet on hemorheological functions in SD rats. Front Nutri. 11, 1408778(2024).
- Wu, Q., et al. Insights into the unique roles of extracellular vesicles for gut health modulation: Mechanisms, challenges, and perspectives. Curr Res Microb Sci. 7, 100301(2024).
- Zhang, H., et al. Effects of bacterial extracellular vesicles derived from oral and gastrointestinal pathogens on systemic diseases. Microbiol Res. 285, 127788(2024).
- Wang, H. X., Wang, Y. P. Gut Microbiota-brain Axis. Chinese Med J. 129 (19), 2373-2380 (2016).
- Huang, S., et al. A cross-tissue transcriptome association study identifies key genes in essential hypertension. Front Genet. 14, 1114174(2023).
- Gao, H., et al. Microbial DNA Enrichment Promotes Adrenomedullary Inflammation, Catecholamine Secretion, and Hypertension in Obese Mice. J Am Heart Assoc. 11 (4), e024561(2022).
- Chen, P. Gut Microbiota and Pathogenesis of Organ Injury. , Springer. Singapore. (2020).
- Xie, N., et al. hPSCs-derived brain organoids for disease modeling, toxicity testing and drug evaluation. Exp Neurol. 385, 115110(2024).
- Liu, N., et al. The underlying mechanisms of DNA methylation in high salt memory in hypertensive vascular disease. Sci Rep. 14 (1), 925(2024).
- Li, M., et al. Wheat β-glucan reduces obesity and hyperlipidemia in mice with high-fat and high-salt diet by regulating intestinal flora. Int J Biol Macromol. 288, 138754(2024).
- Kerem, G., et al. Small intestinal microbiota composition altered in obesity-T2DM mice with high salt fed. Sci Rep. 13 (1), 8256(2023).
- Díez-Sainz, E., Milagro, F. I., Riezu-Boj, J. I., Lorente-Cebrián, S. Effects of gut microbiota-derived extracellular vesicles on obesity and diabetes and their potential modulation through diet. J Physiol Biochem. 78 (2), 485-499 (2022).
- Wu, S., et al. Liuzijue training improves hypertension and modulates gut microbiota profile. Front Cardiovasc Med. 10, 1075084(2023).
- Qi, L. M., et al. Salvia miltiorrhiza bunge extract improves the Th17/Treg imbalance and modulates gut microbiota of hypertensive rats induced by high-salt diet. J Funct Foods. 117, 106211(2024).
- Wilck, N., et al. Salt-responsive gut commensal modulates T(H)17 axis and disease. Nature. 551 (7682), 585-589 (2017).
- Jiang, X., et al. Intestinal Gastrin/CCKBR (Cholecystokinin B Receptor) Ameliorates Salt-Sensitive Hypertension by Inhibiting Intestinal Na(+)/H(+) Exchanger 3 Activity Through a PKC (Protein Kinase C)-Mediated NHERF1 and NHERF2 Pathway. Hypertension. 79 (8), 1668-1679 (2022).
- Zheng, T., et al. Hypertension of liver-yang hyperactivity syndrome induced by a high salt diet by altering components of the gut microbiota associated with the glutamate/GABA-glutamine cycle. Front Nutr. 9, 964273(2022).
- Mulligan, S. K., et al. Multiplexed TEM Specimen Preparation and Analysis of Plasmonic Nanoparticles. Microsc Microanal. 21 (4), 1017-1025 (2015).
- Olson, B. Assays for Determination of Protein Concentration. Curr Protoc Pharmacol. 73, A.3a.1-a.3a.32 (2016).
- Matsumoto, H., Haniu, H., Komori, N. Determination of Protein Molecular Weights on SDS-PAGE. Methods Mol Biol. 1855, 101-105 (2019).
- Sanschagrin, S., Yergeau, E. Next-generation Sequencing of 16S Ribosomal RNA Gene Amplicons. J Vis Exp. (90), e51709(2014).
- Tulkens, J., De Wever, O., Hendrix, A. Analyzing bacterial extracellular vesicles in human body fluids by orthogonal biophysical separation and biochemical characterization. Nat Protoc. 15 (1), 40-67 (2020).
- Ruhal, R., Kataria, R. Biofilm patterns in gram-positive and gram-negative bacteria. Microbiol Res. 251, 126829(2021).
- Maldonado, R. F., Sá-Correia, I., Valvano, M. A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbio Rev. 40 (4), 480-493 (2016).
- Honda, K., Littman, D. R. The microbiota in adaptive immune homeostasis and disease. Nature. 535 (7610), 75-84 (2016).
- Guzik, T. J., et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 204 (10), 2449-2460 (2007).
- Huang, J., et al. Extracellular vesicles as a novel mediator of interkingdom communication. Cytokine Growth Factor Rev. 73, 173-184 (2023).
- Li, W., et al. Alleviation of colitis by honeysuckle MIR2911 via direct regulation of gut microbiota. J Control Release. 376, 123-137 (2024).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved