Method Article
Matrix-based DNA Extraction for Targeted Next-Generation Sequencing on Decontaminated Sputum Samples
* These authors contributed equally
In This Article
Summary
We present an optimized method for DNA extraction from decontaminated sputum using a matrix-based extraction method combined with a magnetic bead purification for downstream targeted next-generation sequencing of Mycobacterium tuberculosis.
Abstract
Next-generation sequencing (NGS) is now recognized as a powerful tool for timely and accurate drug-resistant tuberculosis (DR-TB) diagnosis. Targeted NGS (tNGS) offers a streamlined approach by focusing on specific genes associated with drug resistance, bypassing the need for traditional culture-based methods with turnaround times ranging from weeks to months. The World Health Organization (WHO) has recommended tNGS as a valuable strategy for improving tuberculosis (TB) diagnosis to guide treatment and improve treatment outcomes, particularly in resource-limited settings. Among the WHO-recommended tNGS assays, we have selected a method that provides rapid and comprehensive drug susceptibility testing, lineage determination, and strain typing. While standardized DNA extraction methods are available, they can be time-consuming and labor-intensive. To address this challenge, we optimized a simplified, matrix-based DNA extraction protocol in combination with magnetic bead purification. This method offers a rapid and efficient approach for extracting DNA directly from decontaminated sputum sediments, enabling rapid downstream tNGS analysis. By streamlining the DNA extraction process from sputum sediment, this protocol could facilitate wider adoption of tNGS in routine clinical settings, ultimately contributing to improved patient outcomes and lending to global TB control efforts.
Introduction
An estimated 3.7 million people with TB went undiagnosed and untreated globally in 2023, highlighting the significant threat posed by TB to global health1. The WHO estimates that approximately 400,000 people developed rifampicin-resistant (RR-TB) or multidrug-resistant TB (MDR-TB) in 20231. Diagnosing and treating TB and DR-TB rapidly is essential to achieving the milestones and targets for reducing TB incidence and mortality1.
Relying on conventional culture methods and phenotypic drug susceptibility testing (pDST) delays the determination of the resistance profile of clinical isolates and treatment, with turnaround times of 6-8 weeks, and requires complex biocontainment infrastructure. Routine diagnostic tests, in combination with NGS of DR-TB, can provide a comprehensive drug resistance profile and improve the personalization of MDR-TB regimens, while also reducing the time to effective treatment from weeks or months to days2,3,4.
In 2023 and 2024, the WHO recommended the use of tNGS as a new class of diagnostic to rapidly determine susceptibility to first- and second-line anti-TB drugs5. This makes it a valuable tool for guiding treatment decisions without the need for Mycobacterium tuberculosis (MTB) culture in biosafety level 3 (BSL-3) laboratories. A tNGS approach is a condensed form of sequencing that uses polymerase chain reaction (PCR) to amplify drug-resistance-conferring gene targets prior to sequencing. Among WHO-recommended tNGS tests, we have selected the Deeplex Myc-TB assay, which has been reported to meet the class-based criteria for detecting resistance to rifampicin, isoniazid, ethambutol, pyrazinamide, fluoroquinolones, amikacin, streptomycin, linezolid, bedaquiline, and clofazimine. We use this assay to evaluate the suitability of the DNA extracted using this protocol for downstream tNGS.
Additionally, the WHO published the 2nd edition of the catalog of mutations associated with drug resistance in MTB, providing a roadmap for the use of whole-genome sequencing (WGS) and tNGS to predict drug susceptibility and guide treatment6. A recent systematic review and meta-analysis showed that tNGS had a sensitivity and specificity of 94.1% and 98.1%, respectively, for the detection of drug resistance, based on 23 targets in various resistance-conferring regions in the MTB genome, compared to pDST7.
However, implementation of these methods remains challenging due to complexity and costs specifically associated with the required workflows, infrastructure, and equipment. A critical challenge is isolating sufficient high-quality mycobacterial DNA directly from the sediment of decontaminated sputum, a crucial step for downstream tNGS applications. To address this, we present a rapid and simple DNA extraction method tailored for tNGS.
The standardized DNA extraction method for the selected MTB tNGS assay includes an in-house manual and automated protocol8. Here we describe a simplified, matrix-based, DNA extraction protocol (Figure 1). The method makes use of the InstaGene Matrix (IGM), which binds metals and proteins allowing quality nucleic acid extraction directly from decontaminated sputum sediments. This alternative provides a quicker turnaround time and a sufficient DNA yield for downstream tNGS. This protocol overcomes the complexities of the manual and automated methods while ensuring quality tNGS for the rapid diagnosis of variants that confer resistance in MTB. With the growing interest in using tNGS within the mycobacteriology field, this protocol could facilitate its adoption into routine diagnostic workflows.
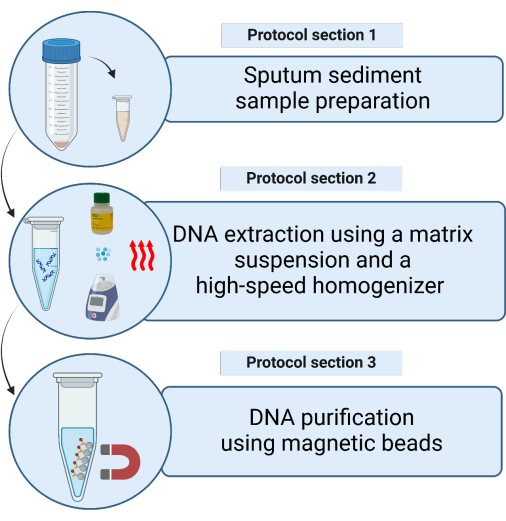
Figure 1: Schematic representation of the methods for extracting mycobacterial DNA from decontaminated sputum sediment samples using a matrix suspension. Please click here to view a larger version of this figure.
Protocol
This research has been approved by Research Ethics Committee: Biological and Environmental Safety (REC: BES) at Stellenbosch University: BES-2024-25384 and Human Research Ethics Committee: N21/09/093 and N09/11/296.
1. Sputum sediment sample preparation
NOTE: The following steps are to be performed in a biosafety level three (BSL3) laboratory prior to DNA extraction.
- Prepare an aliquot of the sputum sediment sample previously decontaminated using N-acetyl-L-cysteine (NALC) and sodium hydroxide (NaOH) (NALC/NaOH) by transferring 500 µL-2 mL to a 1.5 mL or 2 mL low-bind tube.
- Place the tubes containing sputum sediment samples upright in a tube rack and place the samples in an oven at 80 °C and incubate for 1 h to inactivate the mycobacteria through heat treatment.
- After 1 h, remove the tube rack with samples, wipe the tube surfaces, and place them in an appropriate container for transport to a biosafety level two (BSL2) laboratory.
NOTE: Allow samples to cool to room temperature before further handling or storage.
2. DNA extraction using the matrix suspension and a high-speed homogenizer (Figure 2)
- Before starting, add three glass beads of 2 mm diameter to 1.5 or 2 mL screw cap tubes, loosely capped, and autoclave (ideally at least a day before DNA extraction).
- Remove the matrix suspension from the refrigerator and place it on a magnetic stirrer (a magnet is included in the reagent bottle) to bring the reagent to room temperature and start mixing the reagent.
- Switch on the heating block and preheat to 56 °C (if two heating blocks are available, preheat the second to 100 °C.
NOTE: The following steps can be performed on a laboratory bench in a BSL2 facility: - Centrifuge the heat-inactivated sputum sediment sample at 16,800 × g (or full speed) for 15 min. With a 20-200 µL pipette gently aspirate and discard the supernatant, ensuring the pellet remains undisturbed.
- Mix the matrix suspension by gentle shaking (do not vortex), and add 200 µL of the well-mixed matrix to resuspend the pellet.
- Pipette the full volume into a 1.5 mL screw-cap tube containing three presterilized glass beads of 2 mm diameter.
- Place the samples in a heating block and incubate at 56 °C for 15 min.
NOTE: Immediately after removing the sample from the heating block, set the heating block to 100 °C after step 2.7, if only one heating block is available. - Homogenize the samples using a vortex for 10 s to disperse the cells.
- Place the samples in a heating block and incubate at 100 °C for 10 min.
- Homogenize the samples using a high-speed homogenizer with the following parameters: one cycle of 60 s at 4.0 m/s.
- Centrifuge at 12,000 × g for 5 min. Transfer 130 µL of the DNA-containing supernatant to a fresh 1.5 mL low-binding tube without disturbing the pellet (matrix with cell debris). Discard the first tube containing the matrix and cell debris.
NOTE: SAFE STOPPING POINT. If stopping, freeze samples at -20 °C until ready to continue with DNA purification. - Proceed with DNA purification using magnetic beads.
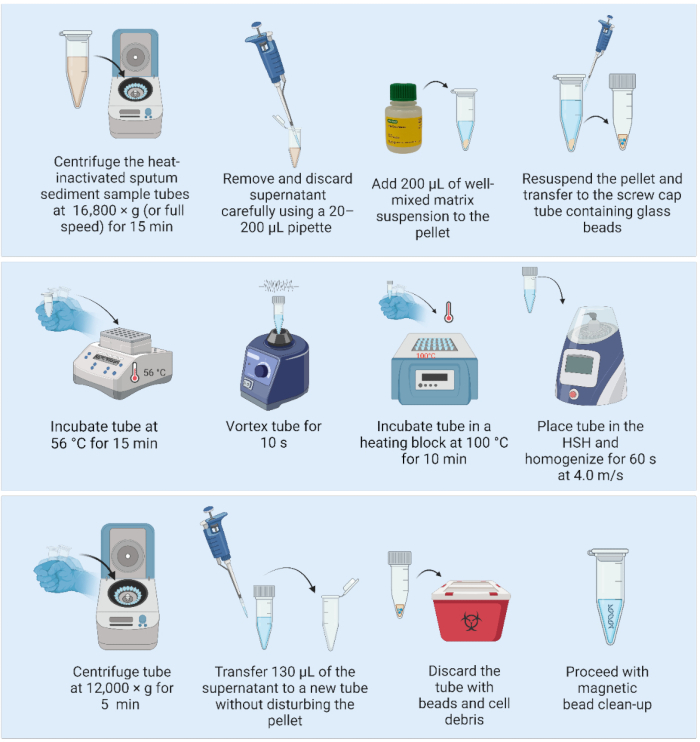
Figure 2: Matrix-based DNA extraction method coupled with a high-speed homogenizer. Abbreviation: HSH = high-speed homogenizer. Please click here to view a larger version of this figure.
3. DNA purification using magnetic beads (Figure 3)
NOTE: Before proceeding with the DNA purification, perform steps 3.1 and 3.2.
- Remove the magnetic beads from the refrigerator and place the container at room temperature for 30 min to equilibrate. Alternatively, vortex the magnetic bead stock bottle to resuspend all the beads and aspirate a 1,280 µL aliquot into a new tube (for purification of 21 samples) and place at room temperature to equilibrate.
- Prepare a 9 mL batch of 80% ethanol by adding 7.2 mL of pure ethyl alcohol to 1.8 mL of filtered and sterilized water.
NOTE: The following steps can be performed on a laboratory bench in a BSL2 facility. - Vortex the magnetic bead stock bottle or the prepared aliquot thoroughly to ensure the beads are resuspended before use. Repeat the vortexing after every 10 samples to ensure a homogeneous suspension.
- Add 1.2x volume (156 µL) of the magnetic beads to the extracted DNA (130 µL).
NOTE: For samples with larger amounts of DNA (culture), a smaller aliquot of the sample can be processed (e.g., 50 µL) and purified with 1.2x volume of magnetic beads. - Mix the samples by pipetting 10x. Incubate the samples for 5 min at room temperature.
- Place the samples on the 1.5 mL tube magnetic rack for 3 min or until the liquid becomes clear. Carefully aspirate and discard the supernatant without disturbing the beads.
- With the tubes on the magnet, add 200 µL of 80% ethanol, ensuring the beads remain undisturbed and allow to incubate for 30 s.
- Carefully aspirate and discard the ethanol without disturbing the beads.
- Repeat step 3.7 for a total of two washes.
- After the second wash, remove and discard the ethanol. Remove any residual ethanol with a 1-10 µL pipette.
- Leave the tubes open to air dry the beads for 10 min, or until the beads have a matte appearance.
NOTE: Do not allow the beads to overdry and become cracked. - Once the beads have a matte appearance, immediately remove the tubes from the magnet and add 50 µL of nuclease-free water (NFW) directly onto the beads in each sample. After adding NFW to all the tubes, mix each individual sample by pipetting 10x. Inspect the tube for any beads that are stuck on the inside of the tube and repeat the mixing step if necessary. Incubate the samples for 5 min at room temperature.
NOTE: Incubation can be increased from 5 min to 10 min, but do not decrease incubation time to less than 5 min. - Place the tubes back on the magnetic rack for 3 min or until the liquid is clear. With the tubes on the magnetic rack, transfer the DNA-containing supernatant to a clearly marked, sterile, low-binding tube. Ensure that beads are not transferred to the supernatant.
NOTE: If beads are visible in the pipette tip during transfer, pipette the supernatant back into the tube on the magnetic rack to separate for an additional 3 min. Do not transfer beads into the new tube.
SAFE STOPPING POINT. If stopping, freeze samples at -20 °C until ready to continue with quantification using an NGS-appropriate method and subsequent PCR amplification using an MTB tNGS assay.
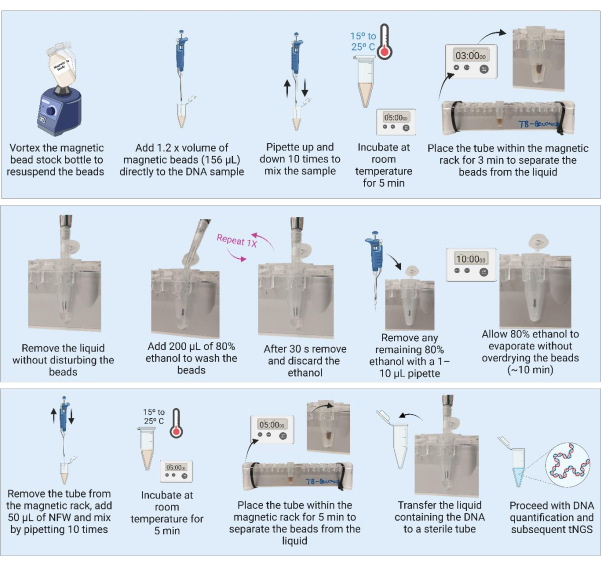
Figure 3: DNA purification and concentration using magnetic beads. Abbreviations: NFW = nuclease-free water; tNGS = targeted next-generation sequencing. Please click here to view a larger version of this figure.
Results
Sample description
A total of 165 sputum sediment samples, positive for acid-fast bacilli (AFB) on microscopy with a bacterial load of at least 1+, were routinely collected and processed by the National Health Laboratory Services (NHLS) Green Point, Cape Town, South Africa. DNA was extracted from sputum sediment samples using two different volumes [approximately 2 mL (n = 102) and 500 µL (n = 63)]. This comparison was conducted to assess whether the smaller sediment sample volume could yield sufficient DNA for downstream tNGS.
Figure 4 shows a boxplot comparison of total DNA concentration (ng/µL) across different sputum smear grades (1+, 2+, and 3+) and sputum sediment volumes (2 mL and 500 µL). DNA yield generally decreases overall when using 500 µL of sediment for extraction, with variability across smear grades. Thus, the mean DNA concentrations extracted from sediment samples with a volume of at least 2 mL were on average higher than the DNA concentration extracted from 500 µL sediments, stratified by AFB of the sample.
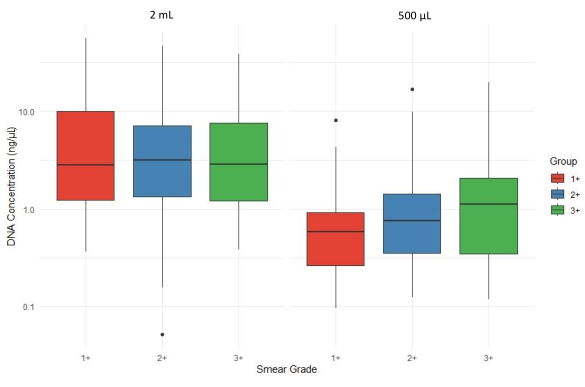
Figure 4: Impact of sputum sediment volume on DNA yield across smear grades. Please click here to view a larger version of this figure.
Figure 5 shows boxplots that illustrate the average number of sequencing reads (average coverage depth) of all targets for sputum sediment samples with varying smear grades (1+, 2+, and 3+) processed from two different input volumes (2 mL and 500 µL). Outliers are represented by individual points, and the y-axis is displayed on a logarithmic scale to accommodate variation in coverage. The high interquartile range for 3+ samples extracted from the 500 µL sediments suggests greater variability with higher bacterial loads.
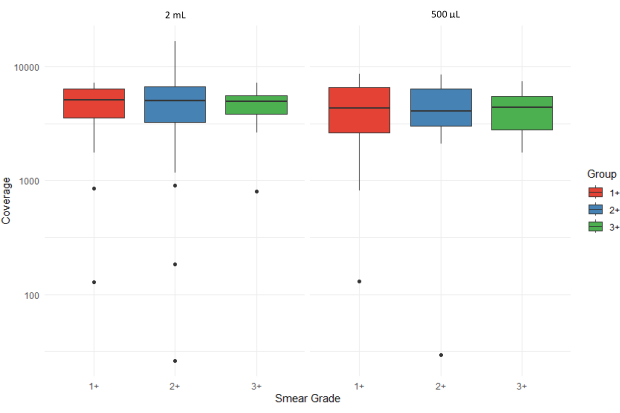
Figure 5: Comparison of average number of sequencing reads (coverage depth) for all targets across smear grades and sample input volumes. Please click here to view a larger version of this figure.
Figure 6 depicts the evaluation of the acceptability of sequencing results using the bioinformatics pipeline of the associated web application. The sequencing quality scores are categorized as +++ (highly acceptable), ++ (acceptable), + (marginally acceptable), - (unacceptable), and ND (not determined). The absence of a bar in the graph indicates that no results were obtained for that specific category. Smear grade 1+ shows a higher proportion of unacceptable and ND results compared to smear grades 2+ and 3+ for the 500 µL input volume sample set. Samples extracted from 500 µL of sputum sediment had an average sequencing coverage depth of 4,316 compared to 4,810 for samples extracted from 2 mL sputum sediment. This suggests that DNA extracted from sputum sediment was adequate for performing downstream tNGS, irrespective of input volume.
Based on the distribution of sequencing result acceptability, samples with a smear grade of 3+ had the highest rate of success with most sample sequences being scored as ++ and +++ compared to 1+ and 2+ smear grade samples. For the 500 µL input samples, the proportion of samples in the - and + categories are relatively higher across all smear grades compared to the 2 mL input samples. This suggests that more samples fall into the lower-quality, less acceptable, categories for the 500 µL input. For the 2 mL input samples, there is a higher proportion of samples in the higher-quality, ++ and +++, categories, particularly for smear grade 3+. This suggests that samples with 2 mL input are more likely to produce better sequencing quality scores compared to those with 500 µL input.
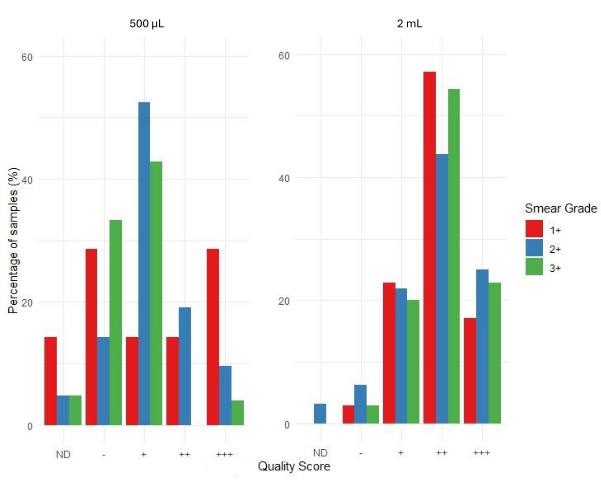
Figure 6: Distribution of quality scores by smear grade and sample input volumes. Please click here to view a larger version of this figure.
Discussion
The combination of the matrix-based extraction method coupled with a high-speed homogenizer was originally published by Shea et al.9. This method was developed and optimized for WGS of DNA extracted from MTBC cultures9,10. We optimized this method for use with an MTB tNGS assay. The DNA extraction and purification of 21 samples had a combined turnaround time of 4 h and 40 min, including incubation and centrifugation steps. The reliability of the described protocol coupled with a bead clean-up for DNA extraction from samples with smear grades varying from 1+ to 3+ is demonstrated based on average yield (Figure 4), the overall depth of coverage of all targets combined (Figure 5), and sequencing result acceptability score as determined by the associated web application (Figure 6).
A recent study found no consistent relationship between smear grade and DNA concentration or sequencing read depth. The authors suggested that variability in sample processing and clean-up steps may affect performance in high smear-grade samples, particularly when contaminants are not efficiently removed. Additionally, sequencing from sputum sediment remains challenging due to the complexity of the DNA pool, where non-target DNA can compete with MTB DNA11. While we did not observe any clear correlation between input DNA concentration and sequencing depth, it is important to note that DNA input before target PCR should not exceed 100 ng, as per the manufacturer's instructions, as this could lead to PCR inhibition. Additionally, higher bacterial load, 2+ and 3+ sputum sediment samples could contain more contaminants, such as human DNA, cell debris, and other inhibitors, which can interfere with PCR amplification and library preparation.
The purification step also functions as a concentration step, enhancing the yield of mycobacterial DNA prior to tNGS. This method is effective for processing samples with a low bacterial load and has demonstrated reliable performance with volumes as small as 500 µL, making it suitable for routine settings where limited sample volume is available. Its application can reduce the need for follow-up visits to clinics, thereby minimizing the risk of patient loss to follow-up. Additionally, the method is simple and does not require advanced laboratory expertise, further supporting its integration into resource-limited settings.
However, some points about the protocol should be noted. Samples should not be frozen or refrigerated immediately after incubation at 80 °C. Rapid cooling immediately following heat treatment can cause condensation to form on the inner surfaces of the tube, leading to dilution of the sample and potentially affecting downstream DNA extraction. Additionally, sudden cooling may increase the risk of sample degradation by promoting nucleic acid fragmentation or enzymatic activity that may not have been completely inactivated during heat treatment. These considerations are particularly relevant when processing sputum samples for MTB detection. According to the WHO recommendations for Ziehl-Neelsen staining, a smear grade of 1+ corresponds to 10-99 AFB per 100 oil immersion fields, 2+ indicates 1-10 AFB per field in at least 50 fields, and 3+ represents more than 10 AFB per field in at least 20 fields12.
A limitation of this method is its lack of automation due to the requirement for manual homogenization using a high-speed homogenizer or bead-beating device. To address this, ongoing efforts are focused on refining the protocol by testing shorter incubation times, lower incubation temperatures, and alternative approaches to high-speed homogenization.
Thus, this matrix-based DNA extraction method, combined with a high-speed homogenizer, is a rapid DNA extraction technique that employs heat and bead beating to release genomic DNA while simultaneously removing PCR inhibitors, such as metals and proteins.
Disclosures
Author T. R. receives funding support from FIND through a service contract with UC San Diego. Author T. R. received grant funding from NIH to develop and evaluate a tNGS solution for drug-resistant TB (R01AI176401). Author T. R. is a co-founder, board member, and unpaid shareholder of Verus Diagnostics Inc.
Acknowledgements
The authors would like to thank the TB laboratory team at the NHLS - Green Point, South Africa for providing technical assistance. This work was supported by the National Institutes of Health (NIH) and the Foundation for Innovative New Diagnostics (FIND), TS ELiOT project Grant ID: R01AI153213, Unitaid Grant ID: 2019-32-FIND MDR. Figures were created using BioRender.com.
Materials
| Name | Company | Catalog Number | Comments |
| Alternative: Benchmark BeadBlaster 24 | Benchmark Scientific | D2400 | |
| AMPure XP Bead-Based Reagent | Beckman Coulter | A63881 | |
| Autoclaved or sterile glass beads (2 mm) | Sigma-Aldrich | Z273627-1EA | |
| Ethyl alcohol, Pure | Sigma-Aldrich | E7023-500ML | |
| Filter pipette tips (10 µL) | Bio-Smart Scientific | FT-10-R | |
| Filter pipette tips (1000 µL) | Bio-Smart Scientific | FT1000-R | |
| Filter pipette tips (20 µl) | Bio-Smart Scientific | FT20-R | |
| Filter pipette tips (200 µL) | Bio-Smart Scientific | FT200-R | |
| Heating-block ranging from 37 °C to 100 °C | Eppendorf | 5382000031 | |
| High-speed homogenizer (HSM) as FastPrep-24 | MP Biomedicals | 116005500 | |
| Incubator 80 °C | Lasec Group | IBLPDHG-9030A | |
| InstaGene Matrix | Bio-Rad Laboratories Inc. | BBRD7326030 | |
| Low-binding microtubes (1.5 mL or 2 mL) | Eppendorf | EP0030108051-250EA | |
| Magnetic rack for 1.5 – 2 mL tubes | Thermo Fisher Scientific Inc. | 12321D | |
| Magnetic stirrer | Merck | Z671886 | |
| Microcentrifuge | Eppendorf | 5406000046 | |
| Milli-Q IX 7015 Pure Water Purification System | Merck | ZIX7015T0C | |
| Nuclease-free water (NFW), molecular grade | Thermo Fisher Scientific Inc. | AM9937 | |
| Pipettes (P10, P20, P200, and P1000) | Eppendorf | EP3123000918-1EA | |
| Qubit Assay Tubes | Thermo Fisher Scientific Inc. | Q32856 | |
| Qubit dsDNA HS Assay Kit | Thermo Fisher Scientific Inc. | Q33231 | |
| Qubit fluorometer instrument | Thermo Fisher Scientific Inc. | Q33226 | |
| Screw cap tubes (1.5 mL) | Scientific Specialties Inc. | P2TUB056C-0001.5ST | |
| Vortex | Lasec Group | WMBB0L0E0216 |
References
- Global Tuberculosis Report 2024. Geneva: WHO. , World Health Organization. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2024 (2024).
- De Araujo, L., et al. Implementation of targeted next-generation sequencing for the diagnosis of drug-resistant tuberculosis in low-resource settings: a programmatic model, challenges, and initial outcomes. Front Public Health. 11, 1204064(2023).
- Murphy, S. G., et al. Direct detection of drug-resistant Mycobacterium tuberculosis using targeted next generation sequencing. Front Public Health. 11, 1206056(2023).
- Kambli, P., et al. Targeted next generation sequencing directly from sputum for comprehensive genetic information on drug resistant Mycobacterium tuberculosis. Tuberculosis. 127, 102051(2021).
- WHO Consolidated Guidelines on Tuberculosis.Module 3: Diagnosis. Rapid diagnostics for tuberculosis detection. Third edition. , World Health Organization. https://iris.who.int/bitstream/handle/10665/376221/9789240089488-eng.pdf?sequence=1 (2024).
- Catalogue of mutations in Mycobacterium tuberculosis. complex and their association with drug resistance. Second edition. , World Health Organization. https://iris.who.int/bitstream/handle/10665/374061/9789240082410-eng.pdf?sequence=1 (2023).
- Schwab, T. C., et al. Targeted next-generation sequencing to diagnose drug-resistant tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 24 (10), 1162-1176 (2024).
- Deeplex Myc-TB manual version 5. , GenoScreen. https://acrobat.adobe.com/link/track?uri=urn%3Aaaid%3Ascds%3AUS%3A67c2b53f-c91d-3a73-a6ea-4769e7c8fe57 (2023).
- Shea, J., et al. Comprehensive whole-genome sequencing and reporting of drug resistance profiles on clinical cases of Mycobacterium tuberculosis in New York State. J Clin Microbiol. 55 (6), 1871-1882 (2017).
- Conceição, E. C., et al. Mycobacterium tuberculosis. DNA extraction using InstaGene Matrix and high-speed homogenizer from clinical primary culture. , https://www.protocols.io/view/mycobacterium-tuberculosis-dna-extraction-using-in-dhvs366e (2024).
- Nilgiriwala, K., et al. Genomic sequencing from sputum for tuberculosis disease diagnosis, lineage determination, and drug susceptibility prediction. J Clin Microbiol. 61 (3), e0157822(2023).
- Laboratory Services in Tuberculosis Control Part II: Microscopy. Geneva: WHO. , World Health Organization. https://iris.who.int/bitstream/handle/10665/65942/WHO_TB_98.258_(part2).pdf?sequence=2 (1998).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved