Method Article
An Adoptive Transfer Model of Rheumatoid Arthritis in Mice
In This Article
Summary
Here, we report a protocol establishing a rheumatoid arthritis (RA) mouse model through adoptive transfer of CD4+ T cells from SKG mice, providing a rapid and reliable experimental tool for investigating the immunological mechanisms, pathological progression, and development of new treatments for RA.
Abstract
Rheumatoid arthritis (RA) is a chronic, systemic autoimmune inflammatory disorder that may result in joint damage, deformities, disability, and even death. Due to its complex etiology and heterogeneous clinical presentation, current treatment strategies remain inadequate in effectively controlling disease progression, particularly in achieving early diagnosis and providing personalized therapies. Therefore, developing novel therapeutic approaches is crucial. To achieve this, reliable animal models are essential for investigating the pathogenesis of RA. Currently, several animal models of RA are used, including the collagen-induced arthritis model (CIA), the K/BxN model, and the SKG mouse. Although these models can successfully mimic the immune mechanisms and clinical manifestations of RA, they each have notable limitations.
In this protocol, we describe the process of establishing an RA mouse model through the adoptive transfer of CD4+ T cells from SKG mice. Compared to conventional models, this model offers a shorter establishment time and a higher incidence rate (100%) in C57BL/6 mice. It is relatively cost-effective, involves straightforward procedures, and reliably replicates T-cell-mediated immune responses, ensuring superior experimental control and reproducibility. We conducted a comprehensive evaluation of the model, assessing the clinical phenotype such as joint symptoms. Through clinical phenotype assessment, we observed significant joint swelling and inflammatory responses. Additionally, using PCR technology to measure the expression levels of key transcription factors, we found that this model effectively simulates T cell-mediated immune responses and key pathological features of RA. With this model, researchers can better simulate the T cell-mediated immune response and key pathological features of RA, thus providing a reliable and effective experimental tool for studying immune mechanisms and pathological progression and developing novel therapies for RA.
Introduction
RA is a chronic, systemic autoimmune inflammatory disease that affects approximately 1% of the global population, causing high morbidity and a heavy socioeconomic burden1,2. The disease is characterized by persistent synovial inflammation, cartilage destruction, and bone erosion, ultimately leading to joint deformities, disabilities, and, in severe cases, premature death3,4,5. The pathogenesis of RA involves the interplay of genetic, environmental, and immune factors, with key features including abnormal activation of cellular immunity, excessive release of pro-inflammatory cytokines, and disruption of immune tolerance6,7. In this process, autoreactive T cells, particularly CD4+ T cells, as key drivers of immune regulation, directly promote the pathological progression of RA through multiple mechanisms.
T helper cell (Th)1 cells produce interferon-gamma (IFN-γ), which activates macrophages and synovial fibroblasts, resulting in the release of tumor necrosis factor (TNF)-α and interleukin (IL)-6 and causing synovitis. Th17 cells secrete IL-17, which promotes the activation of synovial cells and osteoclasts, exacerbating cartilage destruction and bone erosion8,9. Additionally, CD4+ T cells amplify inflammatory responses by activating B cells through co-stimulatory signals, inducing the production of anti-citrullinated protein antibodies (ACPA) and rheumatoid factors (RF)10. Meanwhile, defects in the function and a reduction in the number of Treg cells are key reasons for immune imbalance in RA, leading to uncontrolled inflammation11,12. Due to the complex etiology and heterogeneous clinical presentation, early diagnosis is difficult, and the current treatment of RA is unsatisfactory. Therefore, the development of novel therapeutic approaches is crucial. Reliable animal models are critical for investigating the pathogenesis of RA to gain a deeper understanding of the potential mechanisms of RA and explore new treatment strategies.
Traditional RA models, such as the CIA and K/BxN mouse models13,14,15, contribute to improving our understanding of RA. However, these models show significant limitations. For instance, the CIA model in C57BL/6 mice has a low success rate and long induction period, which diminishes its utility in certain experimental settings. Similarly, although the K/BxN model is valuable, it is costly to establish and has limitations in replicating the complex pathology of human RA, particularly the interactions between immune cells and cytokines.
To address these limitations, we developed a novel RA mouse model by transferring CD4+ T cells from SKG mice with a C57BL/6 background into immunocompetent C57BL/6 mice, in combination with mannan-induced immune activation. This model effectively replicates the key features of RA, including T-cell-mediated immune responses and essential pathological characteristics such as synovial inflammation and joint erosion, while offering advantages in reproducibility, simplicity, and cost-effectiveness. We outlined a detailed methodology for establishing this model, which includes the generation of SKG mice on the C57BL/6 background, isolation of CD4+ T cells from SKG mice, their adoptive transfer, and the subsequent induction by mannan. Additionally, we describe the clinical and histological criteria used to evaluate the severity of arthritis, ensuring its reliability and reproducibility. By consistently mimicking T-cell-mediated immune responses and the fundamental pathological features of RA, this model serves as a robust and efficient experimental tool for investigating the immune mechanisms, disease progression, and the development of new therapies for RA.
Protocol
All experimental procedures in this study strictly followed the guidelines set forth by the "National Institutes of Health Guide for the Care and Use of Laboratory Animals," including animal husbandry, experimental operations, and euthanasia, and were approved by the Animal Ethics Committee of the Tongji Medical College, Huazhong University of Science and Technology.
1. Animals
- Generation of SKG mice (C57BL/6 background)
- Gene editing strategy
- Design a specific guide RNA (gRNA) targeting the ZAP70 gene (gRNA sequence: 5'-CAGCCCACGAGCGAATGCCCTGG-3') and conduct gene editing with the CRISPR/Cas9 system. Design a donor DNA with the ZAP70(W163C) mutation to ensure precise incorporatio-n of the mutation (here, CAGGCCCCACAGGTGGAGAAGCTCATTG
CTACCACAGCCCACGAGCGAATGCCCTG
CTATCACAGCAGCCTGACTCGTGAGGAG
GCCGAGCGCAAACTCTATTCCGGCCA).
NOTE: The gRNA directs the Cas9 protein to the specific site of the ZAP70 gene for precise cutting. The underlined base in the donor DNA sequence indicates mutated bases. This mutation corresponds to the W163C mutation (TGG→TGC) in the ZAP70 protein, which is located in a critical functional domain.
- Design a specific guide RNA (gRNA) targeting the ZAP70 gene (gRNA sequence: 5'-CAGCCCACGAGCGAATGCCCTGG-3') and conduct gene editing with the CRISPR/Cas9 system. Design a donor DNA with the ZAP70(W163C) mutation to ensure precise incorporatio-n of the mutation (here, CAGGCCCCACAGGTGGAGAAGCTCATTG
- Microsyringe injection
- Use microinjection to introduce Cas9 mRNA, gRNA, and donor DNA into fertilized C57BL/6 mouse eggs. Co-inject Cas9 protein and gRNA so they cleave the ZAP70 gene and insert the ZAP70(W163C) mutation via homologous recombination. Transplant the injected fertilized eggs into pseudopregnant female mice, wait approximately 20 days, and designate the mice born as the F0 generation. Identify genotypes through PCR amplification and sequencing (see step 1.1.5.3).
- Genotyping of F0 generation mice
- Perform PCR and sequencing on F0 generation mice to confirm the acquisition of the ZAP70 (W163C) mutation (see step 1.1.5.3).
NOTE: F0 generation mice are chimeric due to rapid embryonic cleavage. They may lack stable genetic transmission. Implement serial breeding to establish stable lines.
- Perform PCR and sequencing on F0 generation mice to confirm the acquisition of the ZAP70 (W163C) mutation (see step 1.1.5.3).
- Acquisition and genotypic identification of F1 generation mice
- Breed F0 positive mice with wild-type C57BL/6J mice to obtain F1 generation mice, and perform genotyping via PCR and sequencing to obtain SKG mice (C57BL/6 background; see step 1.1.5.4).
- PCR-based genotyping methods for F0 and F1 generations
- Excise a 0.5 cm piece from the tail with sterile scissors.
- Extract mouse DNA using an animal genomic DNA extraction kit.
- For F0, prepare the following PCR reaction system: 13.2 µL of ddH2O, 2 µL of PCR buffer, 2 µL of 2.5 mM dNTP, 0.5 µL of each of forward (5'-GATGCCTAGGTGGGTGGGGTTCC-3') and reverse (5'-ACTTGCCTACGCTACTGCTCTACA-3') primers (10 pmol/µL), 0.8 µL of DNA polymerase, and 1 µL of genomic DNA (50-100 ng/µL) extracted from mouse tails, for a final reaction volume of 20 µL. Perform gene amplification using the following PCR program: 94 °C for 3 min; 98 °C for 15 s, 58 °C for 15 s, and 68 °C for 1 min, for 35 cycles; 68 °C for 5 min; hold at 12 °C.
- For F1, prepare the following PCR reaction system: 14.9 µL of ddH2O, 2 µL of 10x Taq PCR buffer, 1 µL of 2.5 mM dNTP, 0.5 µL of each of forward (5'-GATGCCTAGGTGGGTGGGGTTCC-3') and reverse (5'-ACTTGCCTACGCTACTGCTCTACA-3') primers (10 pmol/µL), 0.1 µL of Taq DNA polymerase, and 1 µL of genomic DNA (50-100 ng/µL) extracted from mouse tails, for a final reaction volume of 20 µL. Perform gene amplification using the following PCR program: 94 °C for 5 min; 94 °C for 30 s, 58 °C for 30 s, 72 °C for 1 min, repeated for 35 cycles; 72 °C for 5 min; hold at 12 °C.
- Run electrophoresis to confirm products of the expected size.
- Sequence the PCR products to verify both genotype and the specific mutation.
- Gene editing strategy
- Selection of donor and recipient mice
- Select SKG mice that are 6-8 weeks old (male or female) as donor mice.
- Select C57BL/6 mice that are 6-8 weeks old (preferably female for consistent experimental results) as recipient mice.
- House C57BL/6 and SKG mice (6-8 weeks old) under SPF conditions in individually ventilated cages. Provide free access to SPF-grade feed and sterile water on a 12 h light/dark cycle. Acclimate the mice for 1 week before beginning experiments.
2. Isolation and purification of CD4+ T cells from SKG mice
- Euthanize the SKG mice under a sterile laminar flow hood using CO2 asphyxiation. Immerse the mice in 75% alcohol for 5 min to disinfect. Locate the spleen in the abdominal cavity as well as the lymph nodes (inguinal and popliteal) in the inguinal and popliteal regions. Carefully dissect the spleen and lymph nodes using sterile forceps and scissors and immediately transfer them to prechilled PBS.
- Place the spleen and lymph nodes in separate Petri dishes. Press the tissues through a 70 µm cell strainer using the plunger of a sterile syringe, and gradually add 10-12 mL of prechilled PBS to form a uniform cell suspension. Pass the cell suspension through the same 70 µm cell strainer into a 15 mL centrifuge tube, then centrifuge at 300 × g for 7 min at 4 °C. Discard the supernatant and retain the cell pellet.
- Perform trypan blue staining to assess cell viability. Count the cells and confirm that viability is ≥90%.
- Adjust the cell concentration to 1 × 108 cells/mL with the buffer provided in the CD4+ T cell isolation kit.
- Transfer 100 µL of the cell suspension (107 cells) into a new tube. Add 10 µL of Biotin-Antibody-Cocktail, mix thoroughly, and incubate on ice for 15 min.
- Resuspend the beads by vortexing at maximum speed. Add 10 µL of the Streptavidin bead suspension, mix well, and incubate on ice for 15 min.
- Add 2.5 mL of the buffer provided in the CD4+ T cell isolation kit and place the tube in the magnet for 5 min.
- Carefully pour the liquid (target cells) from the tube into a new sterile tube, then centrifuge at 4 °C, 300 × g for 5 min. Discard the supernatant and retain the cell pellet.
- Add a sufficient amount of sterile PBS solution to adjust the cell concentration to 2 × 106 cells/mL and keep the suspension on ice for later use.
- Use flow cytometry to assess the purity of the sorted cells (Supplemental Figure S1). Calculate cell purity as:(Number of CD4+ T cells / Total cell count) × 100%. Confirm that the purity is ≥90%16.
3. Adoptive transfer of CD4+ T cells
- Anesthetize C57BL/6 mice with 2%-3% isoflurane, reaching a depth of anesthesia where the mice lose all mobility but retain normal spontaneous breathing.
- Gently clean the inner canthus (corner of the eye) of the mouse with a sterile cotton swab to remove secretions and hair, exposing the vein.
- Immobilize the mouse by hand. Draw 200 µL of CD4+ T cell suspension (2 × 105-5 × 105 cells per mouse) into a 1 mL syringe. Insert the needle at a 10-15° angle into the inner canthal vein, ensuring accurate placement. Inject the cell suspension slowly and evenly over 10-15 s.
- After withdrawing the needle, gently press the inner canthal area with a sterile cotton swab for 3-5 s to prevent bleeding.
- Place the mouse in a quiet, dry, and clean cage for monitoring until it fully regains consciousness, with stable breathing and no abnormal behavior (such as twitching or sudden death). Move the mouse to a standard housing cage after confirming stable postoperative status.
NOTE: As the inner canthal vein is small, gentle handling and controlled infusion speed are crucial to avoid vein rupture or cell blockage caused by excessive speed. - Record the infusion details and label the model and control group mice (four animals per group) to prevent confusion in subsequent experiments. Administer CD4+ T cells to model mice, and leave control mice untreated. Ensure all mice are recipient mice.
4. Stimulation and induction of mannan
- Perform mannan induction on day 4 (72 h post CD4+ T cell infusion). Apply the same induction conditions to all experimental and control mice.
- Weigh the mannan powder and dissolve it in sterile PBS to a concentration of 100 mg/mL.
- Hold the mouse correctly to expose its abdomen. Disinfect the skin with 75% alcohol and identify the injection site (~1 cm to the side of the abdominal midline).
- Thoroughly mix the mannan solution and draw the appropriate volume (20-30 mg per mouse) into a 1 mL syringe. Insert the needle at a 45° angle into the peritoneal cavity and inject the solution slowly to ensure even distribution. Withdraw the needle slowly and gently press the injection site with a sterile cotton swab for a few seconds to prevent leakage or infection.
- Place the injected mouse in a quiet and clean cage, and closely monitor for 5-10 min to ensure there is no leakage at the injection site, abdominal distension, or abnormal breathing.
- Record the injection details for each mouse in detail.
5. Measurements
- Observation of clinical phenotype
- Arthritis symptom scoring
- Perform joint assessments every 3 days after mannan induction. Inspect all joints in the forelimbs and hindlimbs, paying special attention to the knee, ankle, wrist, and digit/toe joints. Score the arthritis severity using standard criteria (Table 1)17. Record and compare the total scores at each time point to evaluate the progression of arthritis.
- Arthritis symptom scoring
- Recording of tissue pathological features
- After a month of mannan induction, euthanize the mice under a sterile laminar flow hood using CO2 asphyxiation (following the ethical committee guidelines), secure the mouse on a foam board, and expose the hind limbs. Incise the skin starting at the ankle and peel upward to expose muscles and joints. Identify the ankle joint (between tibia/fibula and talus) and metatarsophalangeal joints (between metatarsals and proximal phalanges) and dissect these joints for histological analysis and prepare paraffin sections.
- Fix the excised tissues in 4% formalin for 72 h, then transfer them to 10% ethylenediaminetetraacetic acid (EDTA, pH 7.4) for 4 weeks of decalcification to ensure complete decalcification.
- After decalcification, wash the tissues with PBS to remove any residual fixative and then dehydrate the tissues through a graded ethanol series (75%, 80%, 95%, 100%).
NOTE: Formalin can irritate the eyes, skin, and respiratory tract. It should be handled in a fume hood. - Embed the tissue in paraffin using a paraffin embedding machine. Use a microtome to section the embedded tissue into 4 µm-thick slices. Float the sections in a 40 °C water bath containing distilled water.
- Transfer and dry the sections. Place the sections on glass slides. Dry them overnight at room temperature (RT). Store the slides at RT for staining.
- Hematoxylin and Eosin (H&E) staining
- Place the slides in a 60 °C oven for 2 h to remove the paraffin.
- At room temperature, immerse the slides in the following sequence for 5 min each: xylene → xylene → 100% ethanol → 100% ethanol → 95% ethanol → 80% ethanol → 75% ethanol. Rinse in distilled water for 2 min.
- Stain the slides sequentially in hematoxylin solution for 5 min, rinse in deionized water for 2 min, then treat with 0.1% ammonia for 1 min, rinse for 3 x 2 min in deionized water, stain with eosin for 1 min, and finally rinse in deionized water for 2 min.
- Immerse the slides for 5 min each in 80% ethanol → 95% ethanol → 100% ethanol → xylene → xylene.
- Air dry the slides naturally and mount with neutral resin.
- Safranin O-Fast Green staining
- Deparaffinize the paraffin sections to water and stain them using a Van Gieson's stain kit. Mount the slides with neutral resin after staining and capture images under a microscope (prepared in step 5.2.5).
- Changes in T cells and cytokines
- Remove the spleen from the mouse using sterile surgical tools.
- Extract total RNA from the spleen tissue using an RNA extraction kit according to the manufacturer's instructions. Quantify the RNA concentration and purity using a spectrophotometer and ensure the A260/A280 ratio is between 1.8 and 2.0.
- Synthesize cDNA from the extracted RNA using a reverse transcription kit. Follow the manufacturer's protocol for the reaction setup and cycling conditions. Store the cDNA at -20 °C for subsequent qPCR analysis.
- Perform qPCR: Measure relative mRNA expression levels of Tbx21, Gata3, Il-17, and Foxp3 in the spleen tissue was measured using SYBR Green-based qPCR in 10 µL reactions containing: 5 µL of 2x SYBR Green master mix, 0.2 µL each of forward and reverse primers (10 µM; sequences in Table 2), 0.5-1 µL cDNA (50-100 ng/µL), and nuclease-free water to volume. Use the following thermal cycling conditions: initial denaturation at 95 °C for 30 s, followed by 40 cycles of 95 °C for 10 s and 60 °C for 30 s, with melt curve analysis (65-95 °C). Normalize the expression levels to Gapdh using the 2(-ΔΔCt) method.
- Serum isolation and cytometric bead array (CBA) analysis
- Collect blood from the mouse using the orbital blood collection by enucleation method, transfer the blood into an anticoagulant-coated collection tube, and let it stand at room temperature for 30 min. Centrifuge the clotted blood at 4 °C, 1,000 × g for 15 min, carefully collect the supernatant (serum), and store it at -80 °C until analysis.
- Determine the expression levels of IL-6, IL-10, IL-17, TNF-α, and IFN-γ in the serum using a CBA kit according to the manufacturer's instructions.
- Remove the spleen from the mouse using sterile surgical tools.
Results
Clinical scoring of joint swelling and incidence rate in mice
Figure 1 shows the clinical scoring of joint swelling and the incidence rate in model mice. The results indicate that all mice in the model group developed the disease (n = 4), with an incidence rate of 100%. The scores in the model group significantly increased, showed brief relief in the second week, and subsequently rose over a period of 6 weeks.
Joint swelling manifestation in mice
The joints of model group mice exhibit significant thickening and swelling, with noticeable swelling and thickening also observed in the forelimbs and hindlimbs (Figure 2).
Pathological results of mouse ankle joints
Pathological results indicate that compared to the control group, the model group mice show significant thickening of the synovium in the ankle joints, discontinuity in bone, and marked aggregation of inflammatory cells (Figure 3, Figure 4, and Figure 5).
Serum T-cell-related inflammatory factors
Compared to the control group, the model group mice showed significant increases in serum levels of IL-10 (7.51 vs 2.15 pg/mL), TNF (78.83 vs 13.11 pg/mL), IL-17 (101.6 vs 12.64 pg/mL), and IFN-γ (5.15 vs 1.90 pg/mL) (p < 0.05). IL-6 also significantly increased compared to the control group (15.59 vs 6.27 pg/mL) (p = 0.05, Figure 6).
Changes in T cell subsets Th1/2/17 and Tregs in the spleen
The mRNA expression levels of Tbx21 (Th1), Gata3 (Th2), Il-17 (Th17), and Foxp3 (Tregs) were measured to assess changes in T cell subsets in the spleen. These genes encode key transcription factors and cytokines that define the differentiation and function of their respective T cell subsets. Compared to the control group, the model group mice showed significantly increased mRNA expression of Tbx21 (1.68 vs 0.61) and Il-17 (26.30 vs 0.75) in the spleen (p < 0.05) relative to the reference gene Gapdh.
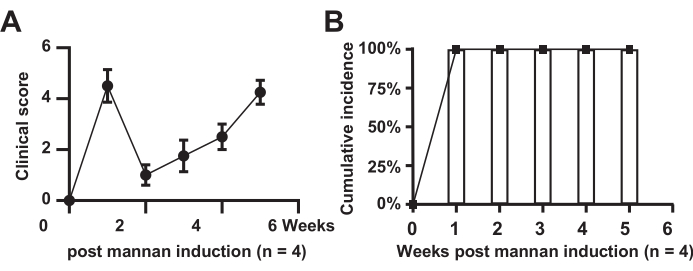
Figure 1: Clinical scoring of joint swelling. (A) Model group mice; (B) Cumulative incidence in the model group mice. Please click here to view a larger version of this figure.

Figure 2: Joint swelling manifestations in control and model mice (n = 4). (A-C) Representative images of paw and joint swelling scores (0,2,4) in the control and model groups of mice. Please click here to view a larger version of this figure.

Figure 3: Histological changes in the ankle joints of control and model group mice observed with hematoxylin-eosin staining. (A) Control mouse ankle joint; (B) Discontinuity in the bone of the model group mouse ankle joint; (C) Synovial proliferation in the model group mouse ankle joint. Scale bars = 50 µm (first column), 100 µm (second and third columns). The images in Fig. 3B and 3C are from the same mouse, demonstrating different pathological phenotypes. Please click here to view a larger version of this figure.
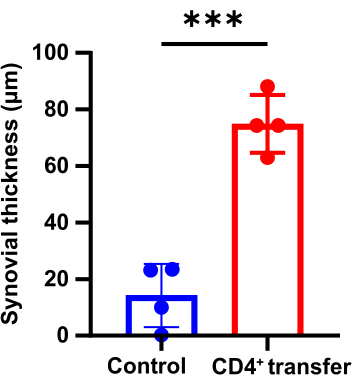
Figure 4: Differences in synovial thickness of the ankle joints between the control group and the model group mice (p < 0.05). Please click here to view a larger version of this figure.
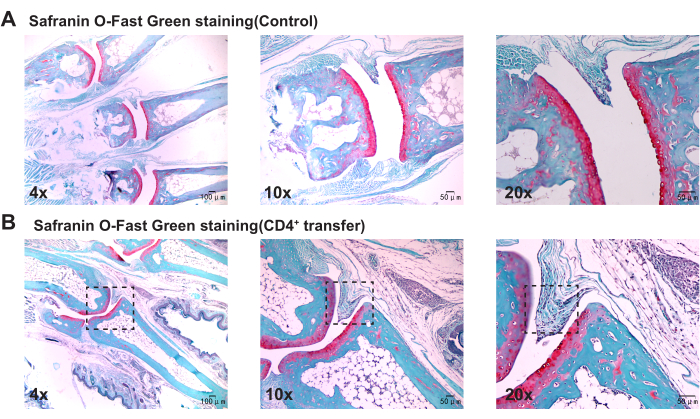
Figure 5: Histological changes in the ankle joints of control and model group mice observed with Safranin O-Fast Green staining. (A) Ankle joint of the control group mouse; (B) Synovial proliferation in the ankle joint of the model group mouse. Scale bars = 50 µm (first column), 100 µm (second and third columns). Please click here to view a larger version of this figure.
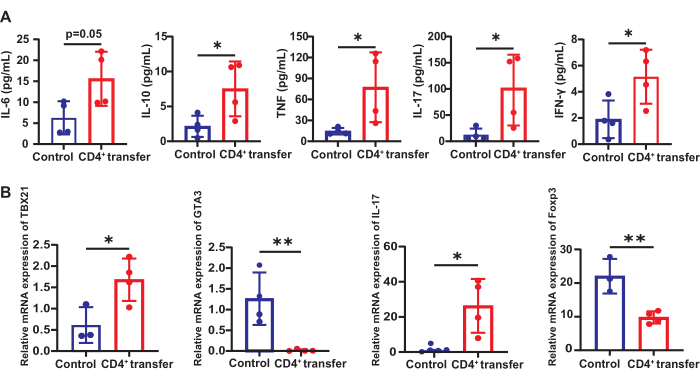
Figure 6: Changes in T cell-related inflammatory factors and subsets in control and model group mice. (A) Expression of T-cell-related inflammatory factors in the serum of control and model group mice and (B) the changes in T cell subsets Th1/2/17 and Tregs in the spleen. *p < 0.05, **p < 0.01. Please click here to view a larger version of this figure.
| Score | Swelling (Range 0-4) | Erythema (Range 0-2) |
| 0 | None | None |
| 1 | Any digit | slight |
| 2 | Paw | Extreme |
| 3 | Wrist/ankle | |
| 4 | Entire limb |
Table 1: Arthritis scoring criteria. To score arthritis, each limb is scored according to swelling and erythema, with a score of 0-6 assigned to each limb. The scores for all four limbs are summed to generate the total arthritis score for each mouse.
| Primer sequences | |
| Oligo Name | Sequence 5' to 3' |
| Tbx21-Forward | AGCAAGGAGCGAATGTT |
| Tbx21-Reverse | GGGTGGACATATAAGCGGTTC |
| Gata3-Forward | CTCGGCCATTCGTACATGGAA |
| Gata3-Reverse | GGATACCTCTGCACCGTAGC |
| Il-17a-Forward | TTTAACTCCCTTGGCGCAAAA |
| Il-17a-Reverse | CTTTCCCTCCGCATTGACAC |
| Foxp3-Forward | CCCTTGACCTCAAAACCAAG |
| Foxp3-Reverse | GTGTGACTGCATGACTAACTTTGA |
| Gapdh-Forward | GGTTGTCTCCTGCGACTTCA |
| Gapdh-Reverse | TGGTCCAGGGTTTCTTACTCC |
Table 2: Primer sequence list.
Supplemental Figure S1: Gating strategy for CD4+ T cell sorting by flow cytometry. Please click here to download this File.
Discussion
The development of RA is closely associated with abnormal immune cell infiltration in the synovial tissue, where the dysregulated activation of these immune cells leads to the release of various pro-inflammatory cytokines, which further contribute to the damage of synovial and joint structures18,19. Several RA animal models have been widely assessed, including the CIA model, the K/BxN model, and SKG mice13,20,21. While these models successfully replicate the immune mechanisms and clinical manifestations of RA, they all have significant limitations. For example, the CIA model in C57BL/6 mice has a lower success rate, a longer onset period, and is significantly influenced by environmental and experimental conditions, making it less practical in certain experimental settings. SKG mice represent an RA mouse model based on a ZAP-70 gene mutation, which causes an abrupt anomaly in T-cell receptor (TCR) signaling and induces autoimmune arthritis22. However, this model is expensive, and the reproducibility of experimental results is easily affected by the induction conditions. K/BxN is a hereditary arthritis model triggered by the cooperative activity of T and B cells, exhibiting a pronounced immune response23. Yet, constructing this model is costly, and its specificity is constrained, leading to a limited immune response that cannot entirely capture the multifaceted pathological process of human RA. Therefore, it is of great importance to develop an animal model that can replicate the key pathological features of RA, meet various experimental requirements, and ensure high reproducibility.
In this study, we present a method for establishing an RA model through the adoptive transfer of adaptive CD4+ T cells from SKG mice. The construction of this model relies on the selection of CD4+ T cells from SKG mice with a C57/BL6 background and the induction of mannan, a process that ensures the success of the model. It is widely known that SKG mice on a BALB/c background carry a spontaneous ZAP70 gene mutation (W163C), which causes abnormal TCR signaling selection, leading to highly self-reactive T cells24. This results in excessive T cell activation and the onset of synovitis and joint destruction, thereby mimicking key pathological processes of RA such as synovial repair and immune cell activation25,26. The clinical features of SKG mice closely resemble those of human RA patients.
To introduce this mutation into the C57BL/6 background, we employed CRISPR/Cas9 technology to successfully generate a C57BL/6-background SKG mouse model carrying the ZAP70 (W163C) mutation. CRISPR/Cas9 offers high precision and efficiency, enabling the targeted introduction of the desired mutation while maintaining a low rate of off-target insertion or unwanted genetic modifications associated with traditional random induction methods, thereby ensuring the model's stability and uniqueness27,28. More importantly, this technique also significantly reduces the time required for model construction, enhancing both the controllability and efficiency of model development. Using this model, we can precisely replicate the T-cell-mediated synovitis and joint destruction of RA against a C57BL/6 background. Compared with the BALB/C background in traditional SKG mice, model mice on a C57BL/6 background have broader applicability in immunological research and can be more easily combined with other transgenic models (e.g., Rag1-/- or IL17-/-). This makes them suitable for investigating the role of the ZAP70 mutation in RA and other autoimmune diseases such as systemic lupus erythematosus or multiple sclerosis.
Based on the pivotal role29,30 of CD4+ T cells in RA activation, we selected them as key mediators. T cells are the primary drivers of immune responses in RA and can directly induce synovial damage by activating Th1/Th17 effector subsets31, depending on inflammatory factors like IL-6, IL-17, and TNF-α32,33. As pivotal mediators of immune regulation, CD4+ T cells secrete pro-inflammatory cytokines, triggering and maintaining the inflammatory cascade, which leads to synovial proliferation and joint damage, thus directly promoting the pathological progression of RA. They are instrumental in aiding B cells in the production of specific antibodies (e.g., ACPA)34. Meanwhile, the typical histopathological feature of the RA synovium is the aggregation of CD4+ T cells. Certain class II major histocompatibility complex (MHC) genes, especially the "shared epitopes" related to Human Leukocyte Antigen-DR isotype (HLA-DR), are considered closely linked to the pathogenesis of RA35. Moreover, the strategy of blocking T-cell co-stimulation with cytotoxic T-lymphocyte-associated antigen 4 (CTLA4)-Ig has demonstrated significant clinical efficacy in RA36. Additionally, mannan induction serves as a potent activator for the model, promoting the activation of innate immune components such as dendritic cells and macrophages37. This step further facilitates CD4+ T cell activation and immune responses, significantly increasing the immune system's attack on self-tissues, thus simulating the pathological features of immune dysregulation and synovial damage in RA.
After completing the model induction, we performed a systematic verification of the model group using multiple parameters, including clinical scoring of joint swelling, pathological validation (observing synovitis tissue pathology and features of joint destruction), and immunological validation (expression of Th1/2/17 and Treg cells in serum and spleen). The results showed that our model successfully replicated T cell-mediated synovitis and joint destruction in RA, inducing characteristic immune activation and synovial pathological changes consistent with the main pathophysiological mechanisms of RA, with a 100% prevalence rate. It is noteworthy that in our model, the clinical swollen-joint score of mice peaks at 1 week, shows a marked reduction in the second week, and then gradually rises again in later stages, which is not entirely in line with the typical course of disease observed in conventional arthritis animal models38. This may be because, at 1 week post induction, the immune system in the model mice is in an intensely activated initial phase by the injection of mannan, resulting in a peak of joint inflammation. In the second week, immune regulation and self-recovery mechanisms lead to a temporary alleviation of clinical symptoms. As the disease progresses, immune tolerance gradually wanes, and the immune response is re-intensified, manifested by a gradual worsening of the condition in later stages.
Although this model is relatively simple compared to other models, there are still several key points that need to be addressed during the modeling process. First, the activity and purity of CD4+ T cells from SKG mice form the basis for the success of the model. The purity of the cells should exceed 90% to ensure the consistency and reliability of the experimental results. Second, the speed of injection into the medial canthal vein should be carefully controlled to avoid vein rupture from injecting too quickly or cell leakage from injecting too slowly. Furthermore, the selection of cell dose is critical. A dose that is too low may lead to model failure, while a dose that is too high could trigger non-specific inflammatory responses. Therefore, during the modeling process, the overall condition of the mice should be closely monitored, and any abnormal symptoms should be recorded promptly to ensure the smooth progress of the experiment and the scientific validity of the data.
Compared to conventional models, this model has a shorter establishment period, achieves a higher incidence rate in C57BL/6 mice, and remains relatively cost-effective and easy to operate. Adopting the transfer of autoreactive CD4+ T cells accurately reproduces T-cell-mediated immune responses and captures key pathological features of RA, such as joint swelling and damage. Moreover, its clinical manifestations align well with those of human RA, offering a more authentic reflection of RA's clinical and pathological processes. Moreover, the combination of medial canthal vein infusion and mannan induction further improves the model's controllability and experimental stability, making it highly reproducible and offering excellent experimental control. Of course, this model still has limitations. It mainly focuses on T cell-driven immune responses; the simulation of the collaborative roles of B cell-mediated antibody responses and other immune cells (such as natural killer (NK) cells and Treg cells) is insufficient, making it challenging to fully represent the multicellular pathological mechanisms of RA. Nonetheless, this RA mouse model remains a stable and reliable animal model, providing researchers with a better platform to simulate T cell-mediated immune responses and the major pathological features of RA. This model allows researchers to delve deeply into the immune mechanisms and pathological progression of RA, providing important experimental evidence and tools for the development of novel therapies, particularly in understanding T cell-driven immune dysregulation and identifying therapeutic targets.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (82270903, 82401588, and 81974254) and China Postdoctoral Science Foundation (2024M751019).
Materials
| Name | Company | Catalog Number | Comments |
| Agarose | Yeasen | 10208ES60 | Preparing agarose gel for use in DNA electrophoresis experiments |
| Anhydrous ethanol | Shanghai HuShi Laboratory Instruments Co., Ltd. | 100009218 | Dehydrating and fixative agent |
| ZAP 70 primers | Tsingke | Primers used for detecting ZAP70 gene expression, commonly used in PCR experiments for genotyping mice | |
| 0.5 M EDTA solution (pH = 7.4) | Biosharp | R00521 | Used for decalcification to ensure the quality of tissue sections and staining for calcium-containing tissues, while maintaining the structural integrity of the tissue |
| 1.5 mL enzyme-free ep tubes | LABSELECT | MCT-001-150-S | Used for a variety of experiments such as sample storage, centrifugal separation, etc |
| 2x Q3 SYBR qPCR Master Mix (Universal) | ToloBio | 22204 | Used for performing highly specific and highly sensitive qPCR reactions. |
| 2x Magic Green Taq SuperMix | TOLOBIO | 21502-04 | Buffer solution for pre-electrophoresis |
| 4% Formaldehyde (paraformaldehyde) solution | Biosharp | BL539A | Used as a fixative to preserve tissue structure and cellular morphology, providing stable and well-preserved samples for subsequent staining steps |
| Animal tissue/cell total RNA isolation kit | Servicebio | MPC2409122 | Extraction of total RNA from animal tissues/cells |
| BD Cytometric Bead Array (CBA) Mouse Th1/Th2/Th17 Cytokine Kit | BD Biosciences | 560485 | Measure the expression of Th1/2/17 cytokines in mouse serum. |
| Cell Culture dish | LABSELECT | 12211 | A container for cell fluid from the spleen and lymph nodes |
| Dimethylbenzene | China National Pharmaceutical Group Chemical Reagents Co., Ltd. | 10023418 | Used as a deparaffinizing and clearing agent |
| Easyfive six-port cell counting chamber | CytoEasy | N3EF110 | Used for cell counting |
| Enhanced Safranin O-Fast Green staining solution for cartilage. | Solarbio | G1371 | Stain cartilage and bone tissues to observe pathological changes in the joint area. |
| Glass slide | CITOTEST | 188105 | Basic support for tissue slicing |
| Hematoxylin staining solution | Servicebio | G1005-1 | Dying |
| Hematoxylin staining solution | Servicebio | G1001 | Dying |
| Low-speed refrigerated centrifuge | Cence | F14300021010004 | It is used for centrifugation and precipitation |
| Mannan | Sigma | m7504 | Activator for the model.Dissolve in sterile PBS at 2 mg/mL, mix thoroughly, and filter through a 0.22 μm membrane to eliminate any particles or possible sources of contamination. |
| MojoSort Magnet | Biolengend | 480019 | Used for isolating and purifying CD4+ T cells from spleen, lymph nodes of mice |
| MojoSort Mouse CD4 T Cell Isolation Kit | Biolengend | 480033 | Used for isolating and purifying CD4+ T cells from spleen, lymph nodes of mice . Use a negative selection method that directly binds to the CD4 molecule to isolate CD4+ T cells, preserving the integrity of surface antigens without affecting CD4 molecule functionality, making it suitable for CD4+ T cell infusion experiments. |
| Nano-300 | ALLSHENG | AS-11020-00 | Accurate detection of nucleic acids, proteins, and cellular solutions |
| Neutral resin mounting agent | Biosharp | BL704A | Fix and preserve stained tissue sections |
| Paraffin microtome | KEDEE | KD-3389 | Used for paraffin sections |
| Phosphate-buffered saline (PBS) | Pricella | WHB824P281 | Used as a buffer solvent or cleaning agent, |
| Sterile cell filter (70 µm) | Biosharp | BS-70-CS | Remove large impurities or aggregated cell clusters from the cell suspension to ensure sample purity and cell uniformity |
| Sterile syringe (1 mL) | Lingyang Medical Apparatus | 20241020 | Injection into the inner canthal vein |
| Tabletop high-speed microrefrigerated centrifuge | SCILOGEX | S1010E | Used for centrifugation and precipitation |
| TEA Buffer (50x) | Yeasen | 60116ES76 | Stabilizes pH, helps protect and preserve molecules like DNA and RNA, used in electrophoresis experiments |
| ToloScript All-in-one RT EasyMix for qPCR | ToloBio | 22107 | Used to convert RNA templates into cDNA, facilitating subsequent gene expression analysis, qPCR, and other molecular biology experiments. |
| Transefer Pipettes | BIOFIL | 240515-133-A | Used for transferring solutions |
| Trypan blue dye solution | Biosharp | 7009529 | Commonly used for cell viability assays, helps differentiate live cells from dead cells |
References
- Global, regional, and national burden of rheumatoid arthritis, 1990-2020, and projections to 2050: A systematic analysis of the global burden of disease study. Lancet Rheumatol. 5 (10), e594-e610 (2021).
- Radu, A. F., Bungau, S. G. Management of rheumatoid arthritis: An overview. Cells. 10 (11), 2857(2021).
- Di Matteo, A., Bathon, J. M., Emery, P. Rheumatoid arthritis. Lancet. 402 (10416), 2019-2033 (2023).
- Bernard, L., et al. Management of patients with rheumatoid arthritis by telemedicine: Connected monitoring. A randomized controlled trial. Joint Bone Spine. 89 (5), 105368(2022).
- Jang, S., Kwon, E. J., Lee, J. J. Rheumatoid arthritis: Pathogenic roles of diverse immune cells. Int J Mol Sci. 23 (2), 905(2022).
- Daikh, D. I. Rheumatoid arthritis: Evolving recognition of a common disease. Best Pract Res Clin Rheumatol. 36 (1), 101740(2022).
- Bhamidipati, K., Wei, K. Precision medicine in rheumatoid arthritis. Best Pract Res Clin Rheumatol. 36 (1), 101742(2022).
- Meyer, A., Parmar, P. J., Shahrara, S. Significance of IL-7 and IL-7R in RA and autoimmunity. Autoimmun Rev. 21 (7), 103120(2022).
- Yu, X., et al. Synergistic induction of CCL5, CXCL9 and CXCL10 by IFN-γ and NLRS ligands on human fibroblast-like synoviocytes-a potential immunopathological mechanism for joint inflammation in rheumatoid arthritis. Int Immunopharmacol. 82, 106356(2020).
- Wu, X., et al. Single-cell sequencing of immune cells from anticitrullinated peptide antibody positive and negative rheumatoid arthritis. Nat Commun. 12 (1), 4977(2021).
- Wei, X., Niu, X. T follicular helper cells in autoimmune diseases. J Autoimmun. 134, 102976(2023).
- Mcelwee, M. K., Dileepan, T., Mahmud, S. A., Jenkins, M. K. The CD4+ T cell repertoire specific for citrullinated peptides shows evidence of immune tolerance. J Exp Med. 220 (12), e20230209(2023).
- Ahmed, S., et al. Dual inhibition of glycolysis and glutaminolysis for synergistic therapy of rheumatoid arthritis. Arthritis Res Ther. 25 (1), 176(2023).
- Wu, J., et al. Tnf antagonist sensitizes synovial fibroblasts to ferroptotic cell death in collagen-induced arthritis mouse models. Nat Commun. 13 (1), 676(2022).
- Huo, F., Hou, J., Zhu, Y., Feng, Z. ferroptosis inducer ike ameliorate pulmonary fibrosis in collagen-induced arthritis (CIA) mice via decreasing the expression of IL-6, CCL5 and CXCL9. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 40 (2), 114-120 (2024).
- Sun, H., et al. ID2 exacerbates the development of rheumatoid arthritis by increasing IFN-γ production in CD4+ T cells. Clin Transl Med. 15 (3), e70242(2025).
- Murayama, M. A., et al. CTRP6 is an endogenous complement regulator that can effectively treat induced arthritis. Nat Commun. 6, 8483(2015).
- Kadura, S., Raghu, G. Rheumatoid arthritis-interstitial lung disease: Manifestations and current concepts in pathogenesis and management. Eur Respir Rev. 30 (160), 210011(2021).
- Mueller, A. L., et al. Recent advances in understanding the pathogenesis of rheumatoid arthritis: New treatment strategies. Cells. 10 (11), 3017(2021).
- Sun, H., et al. Gut commensal parabacteroides distasonis alleviates inflammatory arthritis. Gut. 72 (9), 1664-1677 (2023).
- Li, Z. Y., Zhou, J. J., Luo, C. L., Zhang, L. M. Activation of tgr5 alleviates inflammation in rheumatoid arthritis peripheral blood mononuclear cells and in mice with collagen II-induced arthritis. Mol Med Rep. 20 (5), 4540-4550 (2019).
- Mccarthy, E. E., et al. Endogenous antigens shape the transcriptome and TCR repertoire in an autoimmune arthritis model. J Clin Invest. 135 (2), e174647(2024).
- Chen, J., et al. Annexin A1 attenuates cardiac diastolic dysfunction in mice with inflammatory arthritis. Proc Natl Acad Sci U S A. 118 (38), e2020385118(2021).
- Owada, T., et al. LAT1-specific inhibitor ameliorates severe autoimmune arthritis in skg mouse. Int Immunopharmacol. 109, 108817(2022).
- Zhang, A., et al. Nrf2 activation improves experimental rheumatoid arthritis. Free Radic Biol Med. 207, 279-295 (2023).
- Sakaguchi, N., et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 426 (6965), 454-460 (2003).
- Van Hees, M., et al. New approaches to moderate CRISPR-Cas9 activity: Addressing issues of cellular uptake and endosomal escape. Mol Ther. 30 (1), 32-46 (2022).
- Tyumentseva, M., Tyumentsev, A., Akimkin, V. CRISPR/Cas9 landscape: Current state and future perspectives. Int J Mol Sci. 24 (22), 16077(2023).
- Wu, F., et al. B cells in rheumatoid arthritis: pathogenic mechanisms and treatment prospects. Front Immunol. 12, 750753(2021).
- Dunlap, G., et al. Clonal associations between lymphocyte subsets and functional states in rheumatoid arthritis synovium. Nat Commun. 15 (1), 4991(2024).
- Okamoto, K., Takayanagi, H. Effect of T cells on. Bone. 168, 116675(2023).
- Wang, X. Q., et al. Dopamine D2 receptor on CD4+ T cells is protective against inflammatory responses and signs in a mouse model of rheumatoid arthritis. Arthritis Res Ther. 25 (1), 87(2023).
- Wang, X. Q., Liu, Y., Cai, H. H., Peng, Y. P., Qiu, Y. H. Expression of tyrosine hydroxylase inCD4+ T cells contributes to alleviation of Th17/Treg imbalance in collagen-induced arthritis. Exp Biol Med (Maywood). 241 (18), 2094-2103 (2016).
- Anaparti, V., et al. Increased frequency of TIGIT+ CD4 T cell subset in autoantibody-positive first-degree relatives of patients with rheumatoid arthritis. Front Immunol. 13, 932627(2022).
- Lei, Y., et al. Synovial microenvironment-influenced mast cells promote the progression of rheumatoid arthritis. Nat Commun. 15 (1), 113(2024).
- Jinno, S., et al. Comparison of retention of biologics in Japanese patients with elderly-onset rheumatoid arthritis-the answer cohort study. Rheumatology (Oxford). 64 (2), 509-516 (2025).
- Hagert, C., et al. Rapid spread of mannan to the immune system, skin and joints within 6 hours after local exposure. Clin Exp Immunol. 196 (3), 383-391 (2019).
- Cheng, W. J., et al. Deer velvet antler extracts exert anti-inflammatory and anti-arthritic effects on human rheumatoid arthritis fibroblast-like synoviocytes and distinct mouse arthritis. Am J Chin Med. 50 (6), 1617-1643 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved