Method Article
Minimally Invasive Cisterna Magna Injection Model for Leptomeningeal Metastasis Studies in Mice
W tym Artykule
Podsumowanie
The protocol describes a method for injecting tumor cells through the percutaneous puncture route into cisterna magna that robustly induces leptomeningeal metastasis in mice, reducing trauma and extracranial tumor burden.
Streszczenie
Leptomeningeal metastasis (LM), the spread of cancer cells into the cerebrospinal fluid (CSF)-filled leptomeninges, is a rare yet devastating complication of advanced solid tumors. Patients with LM often have a poor prognosis, with survival measured in weeks to months. Developing in vivo models that accurately replicate the complexities of LM is essential for understanding its cellular and pathological mechanisms and evaluating potential therapies. Murine LM models are typically created through intra-cardiac, carotid artery, or cisterna magna injection of tumor cells. However, intra-cardiac or carotid injections often result in substantial extracranial and brain tumor burden, complicating bioluminescent imaging and leading to mortality unrelated to LM. Meanwhile, conventional cisterna magna injection requires invasive procedures, such as skin incision and muscle dissection, making it both traumatic and resource intensive. Here, we describe a minimally invasive procedure for tumor cell injection into the leptomeningeal space through the cisterna magna without the need for a skin incision. This approach reduces extracranial tumor formation, minimizes surgical trauma, and shortens the time and postoperative care required compared to other surgical methods. Importantly, it consistently induces LM with minimal brain parenchyma infiltration, as confirmed by two-photon microscopy and histological analysis. This streamlined approach offers an efficient and reliable model for studying LM in preclinical research.
Wprowadzenie
The metastatic disease remains the greatest challenge for patients with advanced cancers. Leptomeningeal metastasis (LM) refers to the spread of cancer cells to the pia mater, arachnoid mater, and subarachnoid space. LM from solid tumors is becoming increasingly common in lung cancer (9%-25%), breast cancer (5%-20%), and melanoma (6%-18%)1,2, largely due to longer survival and improved diagnostic techniques. Cancer cells can invade the leptomeningeal space by multiple routes, including 1) direct invasion through peripheral structures such as dura mater, bone, and nerves; 2) hematogenous spread through the venous system; and 3) entry through the arterial circulation, where cancer cells slip through fenestrated vessels into the choroid plexus and subsequently into the cerebrospinal fluid-filled ventricles3,4,5. Tumor cells entering the leptomeningeal space encounter multiple challenges, including deprivation of growth factors, limited metabolic intermediates, and hypoxic conditions6. However, due to the lack of appropriate tools and techniques, how tumor cells navigate these pathways and overcome inhospitable conditions to colonize the leptomeningeal space is poorly understood. Despite advances in multimodal therapies, including radiotherapy, systemic treatment, and intrathecal injection therapy, the prognosis for LM patients remains poor, with survival typically ranging from 2 to 4 months3,7,8,9. Thus, there is an urgent need for a deeper understanding of the biology of leptomeningeal metastasis to improve current treatments and develop novel, targeted therapies. Achieving this requires the development of in vivo models that recapitulate the complex features of LM.
Unlike metastases in organs like the liver, bone, and brain, LM usually develops years after the diagnosis of the primary tumor10,11,12. Similarly, in mouse models with spontaneous metastasis, LM is rare due to its low incidence and the fact that mice typically succumb to metastases at other sites. Experimental murine LM models can be created through various methods, including intracardiac, intracarotid artery, or, alternatively, direct injection into the cisterna magna or cerebral ventricles. While intracardiac injection of cancer cells is widely used9, it often results in a significant extracranial tumor burden, causing mortality unrelated to LM. Alternative approaches, such as injecting tumor cells through the carotid artery13,14, require extensive specialized resources and result in large surgical incisions, which are traumatic. Moreover, this method also primarily leads to metastasis within the brain tissues itself, rather than leptomeninges, and is time-consuming and inefficient for establishing LM models15. Injection into the cisterna magna enables direct delivery of tumor cells to the leptomeningeal space. Several studies have used this approach to investigate LM mechanisms and evaluate new treatments6,16,17.
In this manuscript, we present a convenient trans-cisterna magna injection protocol involving a direct percutaneous puncture to rapidly and stably generate a larger quantity of mice with LM. This method bypasses the brain-blood barrier and, therefore, enables efficient xenograft of tumor cells in the leptomeninges space. It also significantly reduces the surgical trauma and procedure time while reliably inducing LM in mice. We confirmed the occurrence of LM with minimal infiltration into the brain parenchyma, as verified by two-photon microscopy and histological analysis. Therefore, the resulting model faithfully replicates the complex microenvironment of LM, providing a valuable tool to study disease-associated cellular and pathological mechanisms and evaluate potential therapies.
Protokół
All animal procedures in this manuscript were reviewed and approved by the ZJU-Laboratory Animal Welfare and Ethics Review Committee (ZJU20230155). C57BL/6J and NSG mice were obtained from and housed under specific-pathogen-free conditions at the ZJU Laboratory Animal Center. This protocol uses the murine lung cancer cell line, Lewis lung carcinoma (LLC1), and human lung cancer cell line, A549, both labeled with GFP and firefly luciferase. Both cell lines are kindly provided by Dr. Xiang H. F. Zhang (Baylor College of Medicine, USA)18. Here, we use LLC1 cells as an example. The procedure for the injection of A549 cells is almost identical, except that 6 x 104 A549 cells were injected into NSG mice.
1. Preparation of cancer cells for injection
- Culture 1.0 x 106 LLC1 cells in DMEM supplemented with 10% fetal bovine serum (FBS) and 0.1 mg/mL penicillin-streptomycin at 37 °C in a 5% CO2 incubator. When cells reach 70%-90% confluency, trypsinize them for 1 min using 2 mL of 0.25% Trypsin/EDTA solution. Centrifuge the cells at 300 x g for 3 min, wash them 2x with ice-cold PBS, and re-suspend them in 1 mL of phosphate-buffered saline (PBS).
- Assess the concentration of viable cells using a Trypan Blue solution and a hemocytometer19. Ensure the cell viability is greater than 90%, with the majority of cells being single. Adjust the cell concentration to 2 x 106 cells/mL in ice-cold PBS.
- Aliquot 50 µL of the cell suspension into separate microcentrifuge tubes to avoid repeatedly pipetting.
- Keep the cell suspensions on ice until ready for injection. Inject 10 µL of GFP-Luciferase-labeled LLC1 cells per mouse in this protocol, corresponding to 2 x 104 cells per mouse.
NOTE: Adjust the cell number as needed based on the metastatic kinetics of the injected cell lines.
2. Mice preparation
NOTE: In this study, male C57BL/6J mice, aged 6-8 weeks, were used.
- Autoclave all surgical instruments and gloves packed with surgical drapes. Sanitize the benchtop and non-surgical equipment with 75% ethanol, then cover the work area with waterproof drapes.
- Prepare a clean animal housing cage and a warming pad for post-procedure recovery.
- Anesthetize the mouse by subcutaneously injecting 2% tribromoethanol (200 mg/kg). Check the depth of anesthesia using a pinch test before proceeding. Apply sterile ophthalmic ointment to protect the eyes from corneal damage once the mouse is anesthetized.
- Shave the fur from the posterior occipital region using clippers, followed by applying depilatory creams to completely remove the fur in the same region.
- Position the mouse prone with its neck placed over a 15 mL centrifuge tube. Secure the head and lower back with tape and palpate the space between the occiput and C1 vertebra with the index finger (Figure 1).
- Disinfect the posterior occipital region by three rounds of wiping with 75% ethanol-soaked sterile cotton swabs, followed by betadine surgical scrub. Cover the non-sterile parts of the animal with a sterile drape.
3. Cisterna magna injection
NOTE: Aseptic techniques are required for the following steps, including the use of personal protective equipment and sterile gloves.
- Gently pipet the cell suspension and aspirate 10 µL for injection with a 31G, 8 mm insulin syringe.
- Palpate the area between the occiput and C1 of the mouse with index finger to locate the precise puncture site at the lower median margin of the posterior occipital skull. Mark this site if necessary.
- Insert the needle at a 45°-50° angle into the cisterna magna through the identified puncture site, advancing to a depth of 4 mm. A distinct breakthrough sensation indicates that the needle has successfully entered the cisterna magna.
- If the puncture site is difficult to locate, make a small 3-5 mm incision at ear level to expose the posterior midline. If a surgical incision is required, administer Meloxicam (5 mg/kg/day) and Buprenorphine (0.1 mg/kg) subcutaneously 1 h prior to surgery.
- Slowly inject the cell suspension by advancing the syringe plunger, keeping the syringe steady with the hand placed on the table.
- After inoculation, hold the syringe in place for an additional 10 s to allow intracranial pressure to equilibrate. Then, withdraw the needle and press the puncture site with a sterile cotton swab for 1-2 min.
4. Post-injection care
- Transfer the animals to clean cages on a heating pad and monitor them closely until they have fully recovered.
- In case an incision is made, close the wound using tissue glue and clips. Administer additional pain medication for 2-3 days post-surgery to control pain and aid recovery.
- Monitor the mice closely for 7 days following the procedure and check the physical activities of animals and the appearance surrounding the injection site daily.
5. Assessment of leptomeningeal tumor growth
- Bioluminescence imaging
- Anesthetize the mouse and administer D-luciferin (150 µg/g) in the retroorbital vein. Place the mice into the imaging chamber, positioning them in a designated nose cone on the anesthesia manifold. Use light baffles between animals to minimize signal interference.
- Image the animals immediately using the IVIS system with the exposure time between 0.5 s to 2 min6. Confirm successful tumor cell inoculation in the leptomeningeal space by a dispersive bioluminescent signal across the head and spinal cord (Figure 2A).
- Monitor the progression of LM by bioluminescence imaging every 4 days. Adjust imaging intervals based on tumor growth kinetics.
- Histological analysis
- Anesthetize the mice when they were significantly less active or had lost 20% of their body weight and cut the skin and ribs to expose their thoracic cavity. Carefully insert a blunt-tipped cannula into the left ventricle and advance the cannula into the ascending aorta. Perfuse the animal with 20 mL of PBS through the cannula slowly.
- Use scissors to remove the head and make a midline scalp incision to expose the skull. Excise the surrounding soft tissues. Cut along the orbital ridge, then insert scissors into the foramen magnum, and carefully advance along the inner surface of the skull with upward pressure to avoid tissue damage.
- Remove the cranial bones, and then gently extract the brain. Fix the brain in 4% paraformaldehyde at 4 °C for 24 h and then equilibrate it into 15% sucrose PBS solution for 24 h, followed by 30% sucrose PBS solution for another 24 h at 4 °C.
- Place the tissue in a cryomold filled with Optimal Cutting Temperature (OCT) compound and then store it on dry ice for 30 min20.
- Cut the OCT-embedded brain into 10 µm thick sections using a cryostat. Store the sections in a -80 °C freezer until further application.
- Perform Hematoxylin/Eosin (H&E) staining on the brain slides21. The presence of tumor cells at the brain edge indicates that metastasis occurs exclusively in the leptomeningeal space (Figure 3 and Table 1).
- Two-photon microscopy
- Anesthetize the mice with LM. Remove the scalps covering the dorsal skull surface with forceps and scissors. Use a scalpel blade to remove the thin periosteum from the surface of the skull.
- Thin the skull with an abrasive drill until the pial vessels are visible through the thinned skull. Stabilize the observation area of the mouse head with a triangular headpiece secured by tissue adhesive22,23.
- Administer 0.025 mL of 5% (w/v) TRITC-dextran in an infraorbital vein to label the blood vessels22.
- Perform two-photon microscopy through the imaging windows and reconstruct the leptomeningeal space (Figure 4). Detect the bone by second harmonic fluorescence at 450 nm emission with 900 nm excitation24. Visualize GFP-labelled tumor cells and dextran-labelled vessels by collecting fluorescence signals at 507 nm and 572 nm emission with 900 nm and 1000 nm excitation, respectively.
- Stereo fluorescence microscope
- Remove the mouse brain and place it under a stereomicroscope. Visualize GFP-labeled cells using a GFP-specific filter set (Figure 5).
Wyniki
Figure 1 illustrates the placement of the mouse for injection and the puncture site from lateral and front views. Figure 2 shows representative in vivo bioluminescence images of animals tested for generating LM through different approaches. GFP-luciferase-labeled LLC1 cells were injected into the animals through different routes, followed by bioluminescent imaging. As shown in Figure 2A, 10 days after intra-cistern magna injection, the bioluminescent signal was present in the brain of the mouse and distributed along the spinal cord, indicating successful engraftment of tumor cells in the leptomeningeal space. In contrast, intra-carotid artery injections of tumor cells predominantly generated brain parenchymal metastasis with no significant involvement in leptomeninges 21 days post-injection(Figure 2B). For the intra-cardiac injection method, bioluminescence imaging from the supine position shows that most metastases grow in extracranial organs (left), and images from the prone view confirmed that none of the three mice developed LM (right; Figure 2C). The same distribution of tumor cells was observed 10 days after the cistern magna injection of 6 x 104 (10 µL) GFP-Luciferase-labeled A549 cells in NSG mice (Figure 2D), suggesting such an approach robustly generates LM in different mouse strains with different cell lines.
Figure 3 shows representative images of histological and immunofluorescent staining of brain tissues after 14 days of tumor cell injection through the cisterna magna. Hematoxylin-eosin (H&E) staining shows the majority of tumorous areas located in the leptomeningeal space (Figure 3A). Figure 3B shows that most GFP-labeled tumor cells were clustered in the meninges and ventricles, while tumor cells in the parenchyma region are mostly single cells. Figure 4 presents representative two-photon images of the leptomeningeal space of a mouse with LM. The cranial bone (blue) was detected by collecting the second harmonic fluorescence at 450 nm emission with 900 nm excitation24. The vasculature (red) was labeled by TRITC-dextran (70 kDa). GFP-labeled tumor cells (green) were found between the cranial bone (blue) and the brain parenchyma, specifically within the leptomeningeal region. Figure 5 shows the presence of GFP-labelled tumor cells on the brain surface, visualized using a stereo fluorescence microscope. Table 1 displays the comparison between the three methods.
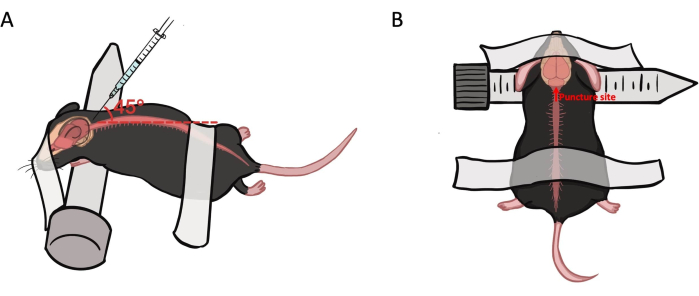
Figure 1: Animal preparation and puncture site for intra-cisterna magna injection. (A) The mouse is placed in a prone position, with its neck draped over a 15 mL centrifuge tube. The head and lower back are secured with tape. The needle is inserted at a 45°-50° angle into the cisterna magna at the median lower margin. (B) The needle is inserted at the median lower margin of the posterior occipital skull. Please click here to view a larger version of this figure.
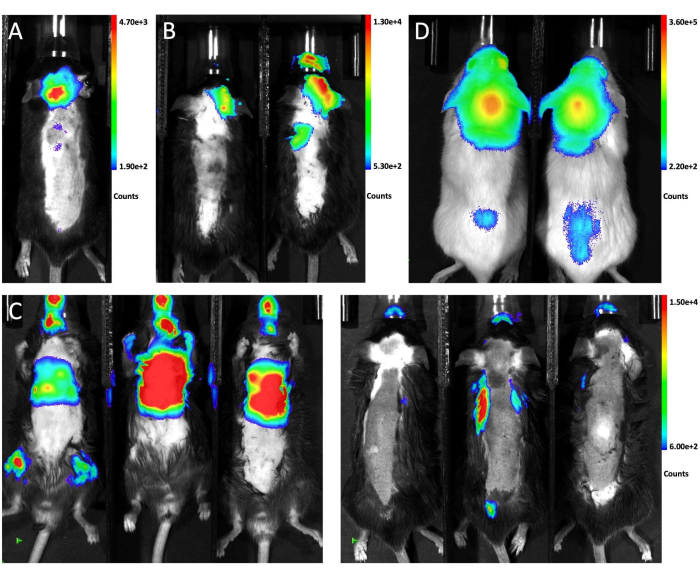
Figure 2: Representative in vivo bioluminescence images of mice receiving different injection methods. (A)The in vivo bioluminescence signal of the whole animal after intra-cisterna magna injection of LLC1 cells.(B) The in vivo bioluminescence image of the mice after intra-carotid artery injection of LLC1 cells. (C) The in vivo bioluminescence signal of the mice after intra-cardiac injection of LLC1 cells. The left side shows the supine view and the right side shows the prone view.(D) The bioluminescence image of NSG mice following the injection of A549 cells through the cisterna magna.a Please click here to view a larger version of this figure.
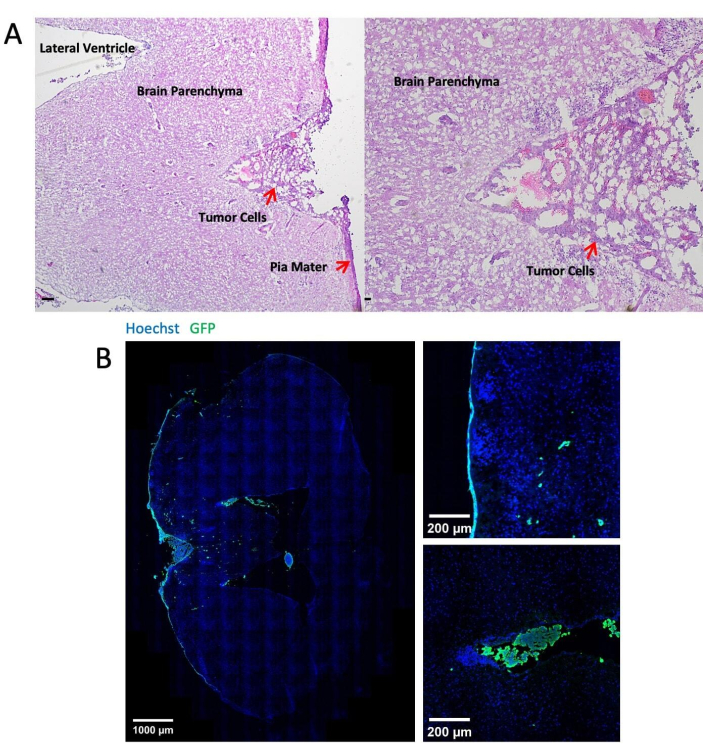
Figure 3: Representative images of histological and immunofluorescent staining. (A) Representative H&E staining shows LLC1 cells deposited mainly on the surface of the meninges and in the ventricles. Scale bar = 25 µm. (B) Immunofluorescent staining images of the LLC1 tumor-bearing brain. Please click here to view a larger version of this figure.
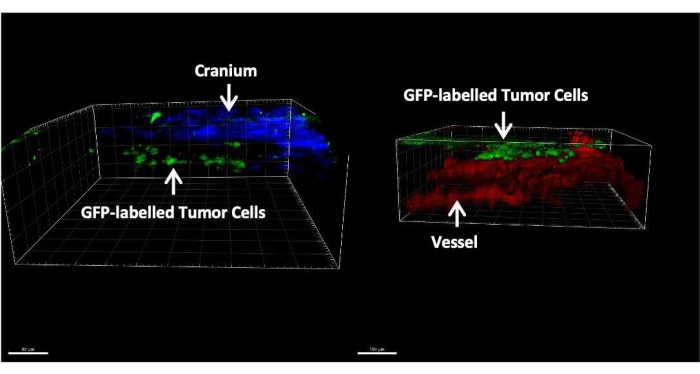
Figure 4: Representative two-photon images of a mouse with LM. Tumor cells labeled with GFP (green) are exclusively found between the skull (blue) and brain parenchyma(red). Please click here to view a larger version of this figure.
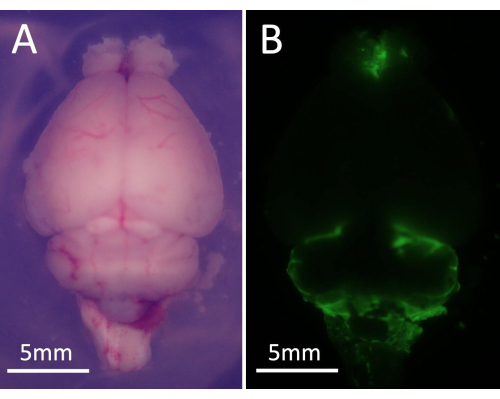
Figure 5: Representative images of the stereo fluorescence microscope. (A) Global visual field of mouse brain under microscope. (B) GFP-labelled tumor cells were presented on the brain surface, which emit green fluorescence. Please click here to view a larger version of this figure.
| Procedure duration/min | Incidence of LM observed 14 days after injection | Rate of extracranial tumor | Rate of large brain parenchymal tumors | |
| Intra-cistern magna injection | ~5 | 100% (15/15) | 0 (0/15) | 0 (0/15) |
| Intra-carotid artery injection | ~20 | 0 (0/15) | 100% (15/15) | 100% (15/15) |
| Intra-cardiac injection | ~5 | 0 (0/3) | 100% (3/3) | 0(0/3) |
Table 1: Comparison of three different injection methods in terms of operation duration, the incidence of leptomeningeal metastasis, extracranial tumors, and the rate of large brain parenchymal tumors.
Dyskusje
LM is an aggressive and fatal condition. Once the tumor cells metastasize to the cerebrospinal fluid-filled space, they rapidly disseminate throughout the entire central nervous system25. These cells settle and invade the brain, spinal cord, cranial, and spinal nerves, ultimately leading to rapid neurological deterioration and eventual death17. To better understand the underlying pathophysiological mechanisms and evaluate potential therapeutic strategies, it is crucial to develop an efficient in vivo model that recapitulates the metastatic process and the surrounding microenvironment in leptomeningeal metastasis.
A previous report has shown that LM can be induced in murine models by directly injecting tumor cells into the right cerebellar hemisphere15. However, this approach often fails to produce metastasis in the subfrontal region or distal spinal cord in some subjects. A recent study demonstrated that tumors derived from mutated small cell lung cancer cells, when implanted subcutaneously, may spontaneously spread to the leptomeningeal space after a prolonged latency period14. Following the in vitro expansion of tumor cells from spontaneous LM, the authors successfully established a cell subline capable of developing LM with minimal brain parenchyma infiltration through intra-carotid artery injection. While this procedure captures the natural course of metastatic dissemination to the leptomeningeal space, it is inefficient and time-consuming, as most mice die from metastases in other organs before LM can fully develop. In addition, this approach necessitates a sophisticated microsurgery, which imposes considerable trauma on the subject26. Alternatively, direct injection of tumor cells into the cisterna magna resulted in extensive leptomeningeal involvement, which closely resembles the condition observed in LM patients15. However, some intracisternal injection methods require skin and muscle dissection, resulting in significant trauma and an increased risk of intracranial infection, which may alter the pathophysiological features of LM.
Here, we described a minimally invasive method for injecting tumor cells into the cisterna magna through a percutaneous puncture route. In contrast to other surgical approaches, percutaneous injections avoid invasive steps such as skin incision, thereby minimizing the risk of infection. With precise positioning, the procedure can be completed in just a few minutes, significantly reducing both operative and postoperative care time. Moreover, direct tumor cell injection into the cisterna magna improves the engraftment of tumor cells within the leptomeningeal space while lowering the incidence of extracranial organ colonization (Table 1).
There are some critical considerations and troubleshooting information for the protocol. Although we expect the current procedure would reduce the need for highly skilled personnel and simplify the whole process, it is still critical for the operator to precisely identify the puncture site. Operators should be familiar with mouse anatomy, particularly the posterior cranial fossa and cervical region, to ensure accurate positioning. If the needle encounters a bony surface during insertion, the puncture site may be too high and should be adjusted downward appropriately. To ensure successful injections, we position and secure the animals above a 15 mL centrifuge tube to fully expose the injection site. We also found that the 31G, 8 mm insulin syringe performs better than the conventional Hamilton syringe for puncturing the cistern magna. This is likely because the Hamilton syringe's needle is less effective at penetrating thick tissue layers and provides less control during insertion. The depth (4 mm) and angle (45°-50°) of needle insertion are crucial. Excessive depth may lead to tumor growth in the brainstem, while insufficient depth may result in subcutaneous tumors. Thus, to minimize the risk of accidental death from a sudden increase in intracranial pressure, the injection should be carried out as slowly as possible.
However, this protocol is not without limitations. Unlike the traditional methods, the procedure described relies on accurate animal positioning and the operator's sensational feedback, which introduces a degree of variability. With careful control of injection depth and repeated practice, a high success rate can be achieved. Another major disadvantage of the current procedure is that it bypasses the early steps of metastatic cascade and, therefore, is not able to recapitulate the whole course of LM in patients. Overall, the presented procedure is technically straightforward and highly efficient for establishing murine LM models, representing a valuable preclinical platform for LM-related studies.
Ujawnienia
The authors declare no conflict of interest.
Podziękowania
The authors thank the Zhang laboratory members for their valuable discussions and assistance throughout this study. W.Z. is supported by the Fundamental Research Funds for the Zhejiang Provincial Universities (2023QZJH60), the Science Fund Program for Distinguished Young Scholars from the National Natural Science Foundation of China (588020-X42306/041), and the startup fund from the Life Sciences Institute of Zhejiang University.
Materiały
| Name | Company | Catalog Number | Comments |
| 1.5ml Eppendorf tubes | Biosharp | BS-15-M-S | |
| 15ml centrifuge tube | LABSELECT | CT-002-15A | |
| 31G x 8mm insulin syringe(0.3ml) | Promisemed | / | |
| Abrasive drill | GLOBALEBIO | GEGZ-AM1 | |
| Animal heat mat | woggee | / | |
| Cryomold | Supin | SP-AB-7 x 7 x 5 | |
| Depilatory creams | Nair | 1.00023E+11 | |
| D-Luciferin | Gold Biology | LUCK-1G | |
| DMEM | Gibco | C11995500CP | |
| FBS | Gibco | 10270-106 | |
| IVIS Spectrum | Caliper | / | |
| Optimal Cutting Temperature | Sakura | 4583-1 | |
| Paraformaldehyde | SCR | 80096618 | |
| PBS | Servicebio | G4202-500ML | |
| Pen/Strep Amphotericin B | Gibco | 15140122 | |
| Shaver | Hipidog | 2103CGMJ3373-GQ22N526 | |
| Stereo fluorescence microscope | Olympus | / | |
| Straight forceps | Beyotime | FS019 | Need to be autoclaved |
| Surgical scissors | Beyotime | FS001 | Need to be autoclaved |
| Triangular mouse fixation head piece | Transcend vivoscope | TVS-FDM-027 | |
| Tribromoethanol | Macklin | C14432922 | |
| TRITC-dextran, MW 70000 | MedChemExpress | HY-158082C | |
| Trypsin/EDTA solution | Gibco | 25200056 | |
| Two-photon laser scanning microscopy | Olympus | / | |
| Vetbond Tissue Adhesives | 3M | 1469SB |
Odniesienia
- Wilcox, J. A., et al. Leptomeningeal metastases from solid tumors: A Society for Neuro-Oncology and American Society of Clinical Oncology consensus review on clinical management and future directions. Neuro Oncol. 26 (10), 1781-1804 (2024).
- Wasserstrom, W. R., Glass, J. P., Posner, J. B. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 49 (4), 759-772 (1982).
- Remsik, J., Boire, A. The path to leptomeningeal metastasis. Nat Rev Cancer. 24 (7), 448-460 (2024).
- Kokkoris, C. P. Leptomeningeal carcinomatosis. How does cancer reach the pia-arachnoid. Cancer. 51 (1), 154-160 (1983).
- Remsik, J., et al. Leptomeningeal metastatic cells adopt two phenotypic states. Cancer Rep. 5 (4), e1236(2022).
- Chi, Y., et al. Cancer cells deploy lipocalin-2 to collect limiting iron in leptomeningeal metastasis. Science. 369 (6501), 276-282 (2020).
- Yin, K., et al. A molecular graded prognostic assessment (molGPA) model specific for estimating survival in lung cancer patients with leptomeningeal metastases. Lung Cancer. 131, 134-138 (2019).
- Li, Y. S., et al. Leptomeningeal Metastases in Patients with NSCLC with EGFR Mutations. J Thorac Oncol. 11 (11), 1962-1969 (2016).
- Li, Y. S., et al. Unique genetic profiles from cerebrospinal fluid cell-free DNA in leptomeningeal metastases of EGFR-mutant non-small-cell lung cancer: a new medium of liquid biopsy. Ann Oncol. 29 (4), 945-952 (2018).
- Posner, J. B., Chernik, N. L. Intracranial metastases from systemic cancer. Adv Neurol. 19, 579-592 (1978).
- Kuiper, J. L., et al. Treatment and survival of patients with EGFR-mutated non-small cell lung cancer and leptomeningeal metastasis: A retrospective cohort analysis. Lung Cancer. 89 (3), 255-261 (2015).
- Tsukada, Y., Fouad, A., Pickren, J. W., Lane, W. W. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer. 52 (12), 2349-2354 (1983).
- Singh, M., Bakhshinyan, D., Venugopal, C., Singh, S. K. Preclinical Modeling and Therapeutic Avenues for Cancer Metastasis to the Central Nervous System. Front Oncol. 7, 220(2017).
- Shi, M. X., et al. PROTAC EZH2 degrader-1 overcomes the resistance of podophyllotoxin derivatives in refractory small cell lung cancer with leptomeningeal metastasis. BMC Cancer. 24 (1), 504(2024).
- Choi, S. A., et al. In vivo bioluminescence imaging for leptomeningeal dissemination of medulloblastoma in mouse models. BMC Cancer. 16 (1), 723(2016).
- Zhao, J., et al. Dura immunity configures leptomeningeal metastasis immunosuppression for cerebrospinal fluid barrier invasion. Nat Cancer. 5 (12), 1940-1961 (2024).
- Boire, A., et al. Complement Component 3 Adapts the Cerebrospinal Fluid for Leptomeningeal Metastasis. Cell. 168 (6), 1101-1113.e13 (2017).
- Zhang, W., et al. Metastasis Initiation Is Coupled with Bone Remodeling through Osteogenic Differentiation of NG2+ Cells. Cancer Discov. 13 (2), 474-495 (2023).
- Louis, K. S., Siegel, A. C. Cell viability analysis using trypan blue: manual and automated methods. Methods Mol Biol. 740, 7-12 (2011).
- Green, T. R. F., Ortiz, J. B., Harrison, J. L., Lifshitz, J., Rowe, R. K. Simultaneous Cryosectioning of Multiple Rodent Brains. J Vis Exp. (139), e58513(2018).
- Feldman, A. T., Wolfe, D. Tissue Processing and Hematoxylin and Eosin Staining. Methods Mol Biol. 1180, 31-43 (2014).
- Shih, A. Y., Mateo, C., Drew, P. J., Tsai, P. S., Kleinfeld, D. A polished and reinforced thinned-skull window for long-term imaging of the mouse brain. J Vis Exp. (61), e3742(2012).
- Shih, A. Y., et al. Two-photon microscopy as a tool to study blood flow and neurovascular coupling in the rodent brain. J Cereb Blood Flow Metab. 32 (7), 1277-1309 (2012).
- Drew, P. J., et al. Chronic optical access through a polished and reinforced thinned skull. Nat Methods. 7 (12), 981-984 (2010).
- Wilcox, J. A., Li, M. J., Boire, A. A. Leptomeningeal Metastases: New Opportunities in the Modern Era. Neurotherapeutics. 19 (6), 1782-1798 (2022).
- Zhang, C., Lowery, F. J., Yu, D. Intracarotid Cancer Cell Injection to Produce Mouse Models of Brain Metastasis. J Vis Exp. (120), e55085(2017).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone