16.11 : 溶解性に影響する因子
イオン性化合物の溶解度は、純水に比べて共通イオン(イオン性化合物の溶解により生成されるイオン)を含む水溶液では小さくなります。これは共通イオン効果と呼ばれる現象の一例で、質量作用の法則の結果をルシャトリエの原理で説明することができます。ヨウ化銀の溶解を例に考えよう。
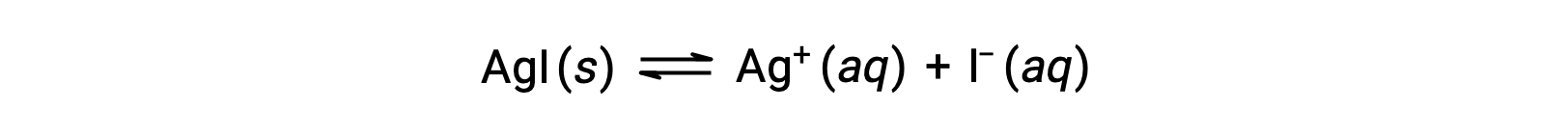
この溶解度の平衡は、銀イオンやヨウ化物イオンの添加によって左にシフトします。その結果、AgIが析出し、溶解したAg+やI–の濃度が低下します。これらのイオンがすでに含まれている溶液では、これらのイオンが含まれていない溶液よりもAgIの溶解量が少なくなります。
この効果は、溶解度積の式に代表されるように、質量作用の観点からも説明できます。
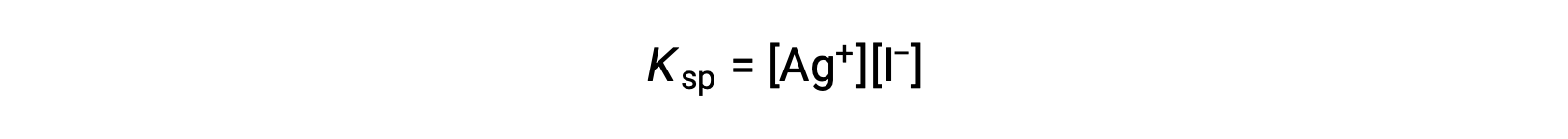
銀イオンとヨウ化物イオンのモル比の積は、イオンの供給源にかかわらず平衡混合物では一定であるため、一方のイオンの濃度が増加すると、それに反比例してもう一方のイオンが減少することでバランスをとる必要があります。
廃水処理における沈殿反応の役割
溶解平衡は、市や町の水道水を処理する施設で行われる廃水処理に有効な手段です。具体的には、自然の水域に戻す前に、廃水から汚染物質を除去するために選択的沈殿が用いられます。例えば、製造施設から排出される水には、リン酸イオン(PO43−)が多く含まれています。リン酸イオンが多いと、藻類が過剰に繁殖し、海洋生物が利用できる酸素量に影響を与えるほか、人間の消費にも適さない水になってしまいます。
水からリン酸塩を除去する一般的な方法は、水酸化カルシウムまたは石灰(Ca(OH)2)を加えることです。水が塩基性になると、カルシウムイオンがリン酸イオンと反応してヒドロキシルアパタイト(Ca5(PO4)3·OH)が生成され、これが溶液中に沈殿します。
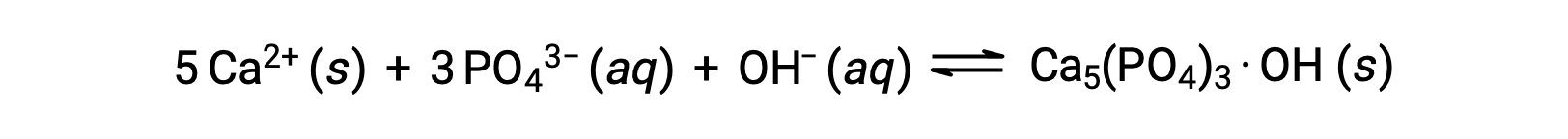
新たに加えたしたカルシウムイオンの量が、他のカルシウム塩の溶解度積を超えないため、それらの塩の陰イオンが排水中に残ます。この沈殿物をろ過して除去し、CO2を添加して再炭酸化することで、水のpHを中性に戻す。沈殿によるリン酸塩の除去には、塩化鉄(III)や硫酸アルミニウムなど、他の化学物質も使用できます。
上記の文章は以下から引用しました。Openstax, Chemistry 2e, Section 15.1: Precipitation and Dissolution.
章から 16:

Now Playing
16.11 : 溶解性に影響する因子
酸塩基と溶解度平衡
33.0K 閲覧数

16.1 : 共通イオン効果
酸塩基と溶解度平衡
40.9K 閲覧数

16.2 : 緩衝液
酸塩基と溶解度平衡
163.3K 閲覧数

16.3 : ヘンダーソン-ハッセルバルヒ式
酸塩基と溶解度平衡
68.0K 閲覧数

16.4 : 緩衝液中のpHの変化の計算
酸塩基と溶解度平衡
52.5K 閲覧数

16.5 : 緩衝液の有効性
酸塩基と溶解度平衡
48.4K 閲覧数

16.6 : 滴定計算:強酸-強塩基
酸塩基と溶解度平衡
28.9K 閲覧数

16.7 : 滴定計算:弱酸-弱塩基
酸塩基と溶解度平衡
43.7K 閲覧数

16.8 : 指示薬
酸塩基と溶解度平衡
47.7K 閲覧数

16.9 : 多価酸の滴定
酸塩基と溶解度平衡
95.6K 閲覧数

16.10 : 溶解平衡
酸塩基と溶解度平衡
51.9K 閲覧数

16.12 : 錯体イオンの形成
酸塩基と溶解度平衡
23.1K 閲覧数

16.13 : イオンの沈殿
酸塩基と溶解度平衡
27.5K 閲覧数

16.14 : 定性分析
酸塩基と溶解度平衡
20.5K 閲覧数

16.15 : 酸塩基滴定曲線
酸塩基と溶解度平衡
126.3K 閲覧数
Copyright © 2023 MyJoVE Corporation. All rights reserved