14.3 : שיווי משקל בתגובות גזיות ובתגובות הטרוגניות
Homogeneous Equilibria for Gaseous Reactions
For gas-phase reactions, the equilibrium constant may be expressed in terms of either the molar concentrations (Kc) or partial pressures (Kp) of the reactants and products. A relation between these two K values may be simply derived from the ideal gas equation and the definition of molarity. According to the ideal gas equation:
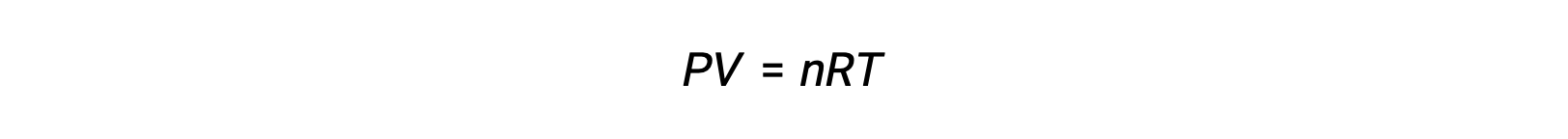
Molar concentration or molarity is given by number of moles divided by the volume:

Thus,

where P is partial pressure, V is volume, n is number of moles, R is the gas constant, T is temperature, and M is molar concentration.
For the gas-phase reaction: m A + n B ⇌ x C + y D

And so, the relationship between Kc and KP is
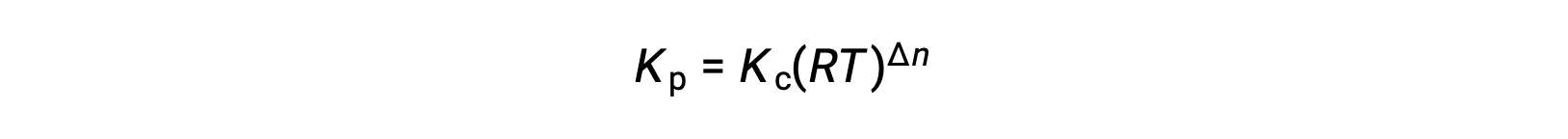
where Δn is the difference in the molar amounts of product and reactant gases, in this case:
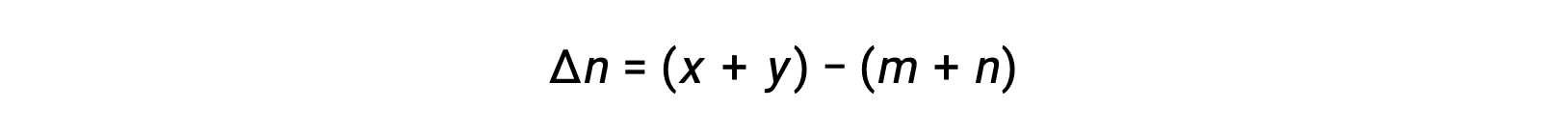
This text has been adapted from Openstax, Chemistry 2e, Section 13.2 Equilibrium Constants.
From Chapter 14:

Now Playing
14.3 : שיווי משקל בתגובות גזיות ובתגובות הטרוגניות
Chemical Equilibrium
24.3K Views

14.1 : שיווי משקל דינמי
Chemical Equilibrium
49.8K Views

14.2 : קבוע שיווי המשקל
Chemical Equilibrium
46.2K Views

14.4 : חישוב קבוע שיווי משקל
Chemical Equilibrium
30.6K Views

14.5 : מנת התגובה
Chemical Equilibrium
47.8K Views

14.6 : חישוב ריכוזי שיווי משקל
Chemical Equilibrium
47.2K Views

14.7 : עיקרון לה שטלייה: שינוי הריכוז
Chemical Equilibrium
57.1K Views

14.8 : עיקרון לה שטלייה: שינוי הנפח (לחץ)
Chemical Equilibrium
33.8K Views

14.9 : עיקרון לה שטלייה: שינוי הטמפרטורה
Chemical Equilibrium
28.8K Views

14.10 : הנחת הx הקטן
Chemical Equilibrium
45.7K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved